Formulary e-News:
Having trouble viewing this email? View in a browser.
|
You are subscribed to %%list.name%% as %%emailaddr%%. Unsubscribe.

|
to Formulary e-News
|
|
 |
|
 |
Survey |
|
Last month’s survey results:
With healthcare reform creating an FDA approval pathway for biosimilar
agents in the United States, what do you see as the primary implications
of this from a managed care perspective in the next 3 years?
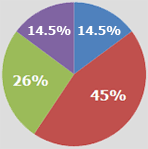
No impact, as I don't see any changes occurring soon: 14.5%
Limited savings with only a small number of products and small number of
manufacturers: 45%
Moderate savings with the introduction of several products at moderate discounting to innovator brands: 26%
Significant savings with several biosimilar options, significant discounts and enhanced rebating from innovator brands: 14.5%
|
|
This month we would like to know...
|
|
How does your health system handle new drug approvals?
1) We have a 6-month moratorium on newly FDA-approved drugs.
2) We have a 12-month moratorium on newly FDA-approved drugs.
3) If approved by the P&T committee, newly FDA-approved drugs can be added at anytime without restrictions.
4) If approved by the P&T committee, newly FDA-approved drugs can be added at anytime with step therapy or other modality to manage drug use and cost.
5) Other

|
|
|
Oseltamivir phosphate oral suspension (Tamiflu) is on back order due to increased demand, manufacturer Genentech announced. Read full article. |
 |
|
An analysis of data from more than 90,000 Medicare managed care enrollees who received care for rheumatoid arthritis found that more than one-third did not receive the recommended treatment with a disease-modifying antirheumatic drug, and that receipt varied by demographic factors, socioeconomic status, geographic location, and health plan, according to a study in JAMA. Read full article.
 |
|
The Advisory Committee on Immunization Practices announces several changes to the recommended adult immunization schedule for 2011. Read full article.
 |
|
FDA has announced that the physician labeling and patient medication guide for rosiglitazone (Avandia, GlaxoSmithKline) have been changed to include information on cardiovascular risks (including death) of this agent. Read full article.
 |
|
Less than 1 month after the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended that most Americans aged 60 years and older get vaccinated to prevent herpes zoster, the vaccine (Zostavax, Merck) is on back order. Read full article.
 |
|
FDA has granted fast-track designation to a novel endothelial cell-based therapy (Vascugel, Pervasis) for the prevention of hemodialysis access failure in patients with end-stage renal disease. Read full article.
 |
THERAPY UPDATE
This article reviews the emerging class of peripherally acting mu-opioid receptor antagonists and provides insight on formulary considerations when evaluating these agents. Read full article.
 |
Top 5 Web Stories
- Plaque psoriasis: A review of recent guidelines and pharmacologic therapies
- Technosphere insulin: An inhaled mealtime insulin for the treatment of type 1 and type 2 diabetes mellitus
- First-time generic approvals January 2011
- FDA Pipeline Preview, January 2011 (Ezogabine, Ticagrelor, Naltrexone SR/bupropion, Clostridium difficile vaccine, Elacytarabine, Glycerol phenylbutyrate, Perifosine, Cladribine Tablets, Florbetapir, ALS-AVP-21D9, Cyclosporine, PRX-8066, QLT091001)
- Pediatric over-the-counter liquids found to have variable and inconsistent dosing directions and measuring devices
 |
|
|
|
Contact Us |
Contact a Formulary editor Click Here
Contact a Formulary sales representative Click Here
Learn about direct mail, reprints and classifieds in Formulary Click Here |
You are subscribed to %%list.name%% as %%emailaddr%%. Click here to unsubscribe or edit your member profile.
To ensure delivery to your inbox, please add us to your address book. If you need help doing this, Click here.
Advanstar Communications provides certain customer contact data (such as customers' names, addresses, phone numbers and e-mail addresses) to third parties who wish to promote relevant products, services and other opportunities which may be of interest to you. If you do not want Advanstar Communications to make your contact information available to third parties for marketing purposes, simply call (toll free) 866-529-2922 at any time, or
fax us at 218-740-6417. Outside the U.S., please phone 218-740-6395. Contact us by mail at Advanstar Communications Inc., 131 West First St., Duluth, MN 55802-2065, USA.
|
|