Formulary e-News:
Having trouble viewing this email? View in a browser.
|
You are subscribed to %%list.name%% as %%emailaddr%%. Unsubscribe.

|
to Formulary e-News
|
|
 |
|
 |
Survey
Last month's survey results:
With several proton pump inhibitors now available OTC, what do you believe is the most appropriate coverage for this class of agents within managed care drug benefit programs?
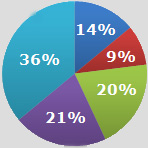
* No coverage should be provided for OTC or prescription products under the drug benefit. 14%
* Coverage of prescription products only under the drug benefit without restriction. 9%
* Coverage of prescription products only under the drug benefits with restrictions (eg, step therapy). 20%
* Coverage of both prescription and OTC products under the drug benefit without restriction. 21%
* Coverage of both prescription and OTC products under the drug benefit with restriction (eg, step therapy). 36% |
|
This month we would like to know...
With the recent introduction of 2 new protease inhibitors as adjunctive therapies for the management of hepatitis C, what do you envision your organization's approach will be to managing the use of these products?
a) Selection of one preferred product only
b) Maintain both agents on formulary and allow providers to choose
c) Employ prior authorization to validate clinical appropriateness before coverage
d) a & c
e) b & c

|
|
|
FDA announced changes to safety labels for simvastatin, Vytorin, and Simcor, including dosing recommendations and is advising physicians to limit the use of 80-mg simvastatin due to an increased risk of myopathy. Read full article. |
 |
To confirm your e-newsletter subscription, click here.
To ensure future delivery of email newsletters from Formulary, please take a moment to confirm your subscription by clicking here.
Thank you,
Formulary Staff
 |
Study shows that several common antiepileptic drugs pose the risk of major congenital malformations and that risk increases dose-dependently. Read full article. |
 |
|
FDA announced reports of medication errors involving risperidone and ropinirole in which some patients who took the wrong medication needed to be hospitalized. Read full article.
 |
|
Safety information regarding varenicline will be added to the Warnings and Precautions section of the label and to the patient Medication Guide because smoking cessation aid varenicline may be associated with an increased risk of certain cardiovascular adverse events in patients who have cardiovascular disease. Read full article.
 |
|
Crizotinib demonstrated an association with a sharp increase in survival rates for patients with advanced non-small cell lung cancer with an anaplastic lymphoma kinase positive genetic alteration, according to the results of a study presented at the annual meeting of the American Society of Clinical Oncology. Read full article.
 |
|
|
|
Contact Us |
Contact a Formulary editor Click Here
Contact a Formulary sales representative Click Here
Learn about direct mail, reprints and classifieds in Formulary Click Here |
You are subscribed to %%list.name%% as %%emailaddr%%. Click here to unsubscribe or edit your member profile.
To ensure delivery to your inbox, please add us to your address book. If you need help doing this, Click here.
 Advanstar Communications provides certain customer contact data (such as customers' names, addresses, phone numbers and e-mail addresses) to third parties who wish to promote relevant products, services and other opportunities which may be of interest to you. If you do not want Advanstar Communications to make your contact information available to third parties for marketing purposes, simply call (toll free) 866-529-2922 at any time, or
fax us at 218-740-6417. Outside the U.S., please phone 218-740-6395. Contact us by mail at Advanstar Communications Inc., 131 West First St., Duluth, MN 55802-2065, USA.
|
|


