|
This month we would like to know...
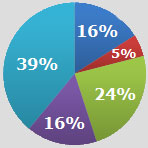
With the evolution of the medical home and ACO models of healthcare delivery, what do you see as the most essential means by which pharmacy departments can support the success of such models?
a) More active direct patient outreach, education, and consultation.
b) Enhanced provider education and drug info support.
c) Improved ability to deliver more timely and actionable patient-specific drug alerts to PCPs/care managers.
d) Enhanced mechanisms to monitor and improve patient compliance.
e) More advanced analytics to better risk stratify patients for intervention need.
f) More timely and robust reporting to measure pharmacy-specific quality performance metrics.

|