Formulary e-News:
Having trouble viewing this email? View in a browser.
|
You are subscribed to %%list.name%% as %%emailaddr%%. Unsubscribe.

|
to Formulary e-News
|
|
 |
|
 |
Survey
Last month's survey results:
With the recent announcement of Medco's acquisition by Express Scripts, potentially bringing 2 of the nation's largest PBMs together, what do you see as the most significant implications of such consolidation for the industry?
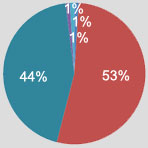
a) Enhanced competition within the industry, inevitably translating to even lower drug costs for PBM payer clients. 1%
b) Unfair advantages for Express Scripts based upon its size, thus reducing competition and potentially increasing long-term costs for payers. 53%
c) Increased pressures on retail pharmacies forcing more pharmacies out of business. 44%
d) Increased quality of PBM clinical, analytic and administrative services across the industry, as other PBMs strive to stay competitive. 1%
e) Minimal, if any, impact. 1%
|
This month we would like to know...
What is your health system’s oncology medication approval process?
a) We follow the CMS-approved compendia. If a medication is listed, it is approved. We do not follow any pathway.
b) We follow the CMS-approved compendia, but a medication is only approved when it follows a pathway.
c) We follow internal guidelines. No pathway is required.
d) We follow internal guidelines, with only specific pathways.
 |
|
|
FDA remains concerned about the potential increased risk of blood clots with the use of drospirenone-containing birth control pills, but it is informing the public that it has not yet reached a conclusion, according to an agency announcement. Read full article. |
 |
Healthcare professionals have access to 2 new online tools that will help them easily compare the effectiveness, benefits, and harmful effects of different treatment options, according to a recent press release from the developers. Read full article. |
 |
ADVERTISEMENT
Resource Center: Oral Anticoagulation in Atrial Fibrillation - Atrial fibrillation afflicts more than 2.3 million persons in the United States and is expected to increase 2.5-fold by 2050. To find risk stratifying tools and articles on emerging oral anticoagulant therapies,
 click here... click here...
 |
|
Everolimus tablets (Afinitor, Novartis) plus the hormonal therapy exemestane more than doubled progression-free survival and significantly reduced the risk of cancer progression by 57% versus exemestane alone for women with advanced breast cancer, according to the results of a phase 3 study presented at the 2011 European Multidisciplinary Cancer Congress in Stockholm, Sweden. Read full article.
 |
|
Investigational drug QTI571 (imatinib) from Novartis significantly improved walking distance in patients with life-threatening pulmonary arterial hypertension, according to the results of a phase 3 study presented at the European Respiratory Society Annual Congress in Amsterdam, The Netherlands. Read full article.
 |
|
In a cohort of 15,417 patients, medication adherence estimates were inflated by 9% to 18% when early nonadherent patients are omitted from adherence calculations, according to results of the study published in the August 18 issue of Annals of Pharmacotherapy. Read full article.
 |
|
|
|
FDA has approved clindamycin injection, USP (Sagent Pharmaceuticals/Strides Arcolab), an antibiotic used to treat bacterial infections. Read full article.
 |
|
FDA and other regulatory and international partners have completed the International Internet Week of Action (IIWA), a cooperative effort to curb online sales and distribution of counterfeit and illegal medical products. Read full article.
 |
|
Blue Shield of California will no longer pay for the use of bevacizumab (Avastin, Genentech, a member of the Roche Group) to treat metastatic breast cancer, according to The New York Times. Read full article.
 |
|
|
|
Contact Us |
Contact a Formulary editor Click Here
Contact a Formulary sales representative Click Here
Learn about direct mail, reprints and classifieds in Formulary Click Here |
You are subscribed to %%list.name%% as %%emailaddr%%. Click here to unsubscribe or edit your member profile.
To ensure delivery to your inbox, please add us to your address book. If you need help doing this, Click here.
 Advanstar Communications provides certain customer contact data (such as customers' names, addresses, phone numbers and e-mail addresses) to third parties who wish to promote relevant products, services and other opportunities which may be of interest to you. If you do not want Advanstar Communications to make your contact information available to third parties for marketing purposes, simply call (toll free) 866-529-2922 at any time, or
fax us at 218-740-6417. Outside the U.S., please phone 218-740-6395. Contact us by mail at Advanstar Communications Inc., 131 West First St., Duluth, MN 55802-2065, USA.
|
|


