
|
 |


 |

 |
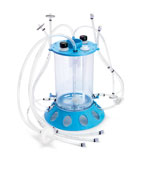 Single-use bioreactor facilitates scale-up Single-use bioreactor facilitates scale-up
The Mobius CellReady 3-L single-use bioreactor, codeveloped by Millipore (Billerica, MA) and Applikon Biotechnology (Schiedam, The Netherlands) brings disposable technology to the process-development scale. The rigid, injection-molded model resembles the glass and stainless-steel bioreactors familiar to biopharmaceutical scientists. The unit’s traditional stirred-tank design provides predictability during scale-up, and its agitation and mixing methods are similar to those of standard bioreactors. In addition, the machine’s marine impeller facilitates oxygenation, increases bubble time from sparging, and is gentle to cells.
The product’s universal design is compatible with most benchtop-bioreactor control systems. Scientists can implement the device immediately and feel comfortable with it quickly, says Andrew Bulpin, Millipore’s vice-president of upstream processing. Because the unit is disposable, the bioreactor decreases downtime compared with traditional glass bioreactors and reduces the time required for cleaning, assembly, and sterilization.
|
|
|
Advertisement:
EMC and RWD
Many Life Sciences companies are exploring new ways to make the most
of valuable but limited patent window of drugs or medical devices they
manufacture.
 To learn more download our webcast To learn more download our webcast
|
|

Catalent Pharma Solutions (Somerset, NJ) created a new business unit that focuses on bringing new pharmaceutical and biologic products to market. The Development and Clinical Services segment brings together Catalent’s analytical and science services and regulatory consulting services, formerly in its Sterile Technologies segment, with its clinical supply services business, formerly included in the Packaging Services segment. Scott Houlton was appointed group president for the new segment, effective August 31st.
In a separate statement, Catalent announced the completion of the first FDA general current good manufacturing practice audit and preapproval inspection of its Buenos Aires, Argentina, pharmaceutical softgel facility, with no reported observations on FDA Form 483. Catalent has been operating in Argentina since 1953 serving local and regional pharmaceutical and consumer health markets and is completing a substantial expansion of the facility designed to meet FDA and other global regulatory standards.
CSL Biotherapies (King of Prussia, PA), a subsidiary of CSL Limited, announced today that the US Food and Drug Administration has licensed the company's newest vaccine filling and packaging facility, located in Kankakee, Illinois. The facility includes a high-speed, single-dose vaccine syringe-filling line. In a separate announcement, the company reported the initiation of its first US clinical trial for its candidate influenza A (H1N1) 2009 vaccine. Study investigators administered vaccinations to volunteers on Aug. 24, 2009, to determine the safety of CSL’s candidate vaccine and its ability to elicit an immune response in adults and children. The pediatric study will evaluate the candidate vaccine in a thimerosal-free formulation.
Eli Lilly announced that the US District Court for the Eastern District of Michigan has granted a motion by Sun Pharmaceuticals for partial summary judgment. The court's ruling invalidates Lilly's '826 patent, or method-of-use patent, for Gemzar (gemcitabine HCl for injection) which had been set to expire in 2013. The ruling has no bearing on Gemzar's compound patent, which remains valid until November 2010. Lilly said in a press release it plans to appeal the ruling with the Court of Appeals for the Federal Circuit.
The biopharmaceutical companies MediciNova (San Diego, CA) and Avigen (Alameda, CA) formed a merger agreement, under which MediciNova's wholly-owned subsidiary will merge with and into Avigen. The transaction will allow the companies to combine their neurological clinical development programs based on ibudilast (Avigen's AV-411 and MediciNova's MN-166). The agreement has been approved by both companies' boards of directors.
Biopharmaceutical company Inovio Biomedical (San Diego, CA) entered into a research collaboration agreement with the National Institutes of Health’s Vaccine Research Center (VRC) to develop influenza vaccines. VRC and Inovio will develop universal influenza vaccines as well as advance development of vaccine candidates targeting the emerging pandemic 2009 H1N1 swine flu strains.
The Procter & Gamble Company (P&G, Cincinnati, OH) agreed to sell its global pharmaceuticals business to specialty pharmaceutical company Warner Chilcott (Ardee, Ireland) for an up-front cash payment of $3.1 billion. Under the terms of the agreement, Warner Chilcott will acquire P&G's portfolio of branded pharmaceutical products, prescription drug product pipeline, and manufacturing facilities in Puerto Rico and Germany. In addition, the majority of the 2300 employees working on P&G's pharmaceuticals business are expected to transfer to Warner Chilcott, according to a P&G press release. Both companies expect the transaction to close by the end of the 2009 calendar year, pending necessary regulatory approvals.
The specialty pharmaceutical companies Santhera Pharmaceuticals (Liestal, Switzerland) and Biovail (Mississauga, Canada) signed a license agreement for Santhera's JP-1730/fipamezole. Biovail acquires the US and Canadian rights to develop and commercialize the drug for the treatment of Dyskinesia in Parkinson's Disease. JP-1730/fipamezole is a compound that, in a recent Phase IIb study, displayed the potential to reduce levodopa-induced dyskinesia.
Transcept Pharmaceuticals (Richmond, CA) will reduce operating expenses by eliminating 30% of its workforce following its recently announced license and collaboration agreement with Purdue Pharmaceutical Products (Stamford, CT) to commercialize Intermezzo (zolpidem tartrate sublingual tablet) in the United States. The company expects to record a restructuring charge of approximately $525,000 in the third quarter of 2009, representing cash payments for severance expenses, the majority of which will be paid in the third and fourth quarters of 2009.
UCB (Brussels) and Novartis (Basel) entered into a licensing agreement for cardiovascular and diabetes products in Germany. UCB extended commercial rights for the German market to the cardiovascular medication Provas (valsartan) beyond 2011 and has licensed in commercial rights for two line extensions, Dafiro (valsartan and amlodipine) and Dafiro HCT (valsartan, amlodipine and HCT). In addition, UCB will cocommercialize the two new oral anti-diabetics Jalra and Icandra (vildagliptin and vildagliptin + metformin).
XSpray Microparticles (Stockholm, Sweden), a life-science technology company, launched a new good-manufacturing-practices production facility in Malmö, Sweden. The company can now provide drug particles and powders for use in clinical studies, as well as offering particle development and characterization services.
Do you have news to report on facility expansions, contracts, service agreements, mergers, acquisitions, or personnel appointments? Send press releases to ptpress@advanstar.com
| |

The US Food and Drug Administration issued Warning Letters to eight companies marketing unlawful over-the-counter (OTC) topical drug products containing the pain-reliever ibuprofen, according to an Aug. 20, 2009 release. The agency allows some OTC drugs to be marketed without first obtaining approval through a new drug application (NDA), but such products must comply with applicable monographs. Because there is no OTC ibuprofen monograph, companies marketing these drugs must submit an NDA. The products and companies receiving Warning Letters are: Emuprofen (Progressive Emu), BioEntopic 15% Ibuprofen Crème (BioCentric Laboratories), Ibunex Topical Ibuprofen (Core Products International), LoPain AF 15% Ibuprofen Crème (Geromatrix Health Products), IB-RELIEF (MEKT LLC), Profen HP (Ridge Medical Products) IbuPRO-10 Plus, (Meditrend,dba Progena ProfessionalFormulations), and IBU-RELIEF 12 (Wonder Laboratories).
Health Canada stated in Aug. 20, 2009 media advisory that it is working with manufacturers to further strengthen product labeling for tumor necrosis factor (TNF) blockers. These particular blockers, used to treat chronic inflammatory diseases, can increase the risk of certain cancers in children and young adults, according to the advisory. The revised labeling requirements will ask manufacturers to note the risk of specific types of cancers that can affect younger patient groups when taking these products. FDA took similar action in early August.
The Philippine government signed a law to strengthen its food and drug administration, according to an Aug. 18, 2009, release from the Office of President Gloria Macapagal-Arroyo. The Republic Act 9711, also known as the Food and Drugs Administration (FDA) Act of 2009, officially renamed the country’s Bureau of Food and Drugs to the Food and Drug Administration and strengthened the agency’s authority. Increased authority under the Act will allow the Philippines’ FDA to “order the ban, recall and withdrawal of health products that cause or has the potential to cause death, serious illness or injury to people,” according to the release. “It can also do the same to products that make deceptive claims.”
The World Health Organization said that orders for the H1N1 vaccine have topped one billion, according to an Agence France-Presse report. For some countries, such as Germany, the United States, Britain, and France, shortages could be a concern because the orders placed would cover roughly 30–78% of the population, according to the report. Other countries such as Greece, The Netherlands, Canada, and Israel, ordered enough of the vaccine to treat their entire populations. "In the early days, there will be a very limited supply of vaccine. There won't be sufficient supply to vaccinate whole populations, or even huge proportions of populations," WHO spokeswoman Melinda Henry told AFP.
The United States Pharmacopeial (USP) Convention signed a Memorandum of Understanding (MOU) with the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) to collaborate on the development of standards for medicines. The MOU allows for the revising, updating, and integrating monographs for medicines contained in both pharmacopeias; exchanging scientific and technical information though meetings, courses, and conferences; and establishing joint reference materials. |
|

AAIPharma Services (Wilmington, NC), a provider of pharmaceutical product-development services, appointed Christopher Smith vice-president of quality and regulatory affairs. The appointment is AAIPharma Services’s first since partnering last month with Water Street Healthcare Partners to become an independent company from AAIPharma Inc.
Amylin Pharmaceuticals (San Diego, CA) appointed Paulo F. Costa chairman of its board. Costa was formerly president and CEO of Novartis US (New York). He was elected to Amylin's board at the company's annual meeting of stockholders on May 27, 2009.
Aureon Laboratories (Yonkers, NY), a specialized laboratory focused on predictive pathology, promoted Robert Shovlin from the company's president to president and CEO. Vijay Aggarwal will step down as CEO but will remain a member of the company's board of directors.
The biotechnology company GENova Biotherapeutics (New York) named Aaron Whiteman its CEO. Whiteman previously worked at companies such as Pfizer (New York) and Amgen (Thousand Oaks, CA).
The RoviSys Company (Aurora, OH), a process-automation integrator, promoted James Riccardi to managing director of its newly opened project support office in Singapore. Riccardi is responsible for project management and client development.
Xcelience (Tampa, FL), a contract research organization, appointed Brett F. Truitt team leader of formulation development in its Pharmaceutical Development Services division. Truitt has more than 10 years experience in pharmaceutical research and formulation development and previously worked at Bilcare Global Clinical Supplies (Phoenixville, PA).
|
|
 |
|
|
|
|
 |

|
FDA’s Enforcement Plan
What do you think of FDA’s recently outlined six steps that the agency will take to increase enforcement of its regulations?
|

View the poll archive. |
|
|
 |


