|
|
 |


 |

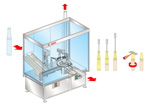 Ampul-filling machine reduces particle generation Ampul-filling machine reduces particle generation
Bosch’s ARF 1010 and ARF 1020 machines can process open and closed ampuls, or open ampuls in combination with vials. The units perform the entire filling and closing process—customers do not need a separate machine to overcap vials. The ARF 1010 unit has a one-position filling station, and a version with a two-position filling station also is available.
Containers are suspended during transport in the starwheel. This design ensures that transport is gentle and protects vials from scratches and cosmetic damage. At the closing station, a special holder rotates injection vials for closing, rather than rotating the vials’ caps. This feature, combined with the gentle transport, reduces particle generation.
The unit’s digital flow meter is designed to ensure the reproduction of flame regulation for the closing of the ampuls. The flame is ignited automatically and can be activated easily from the machine’s operator interface. Digital adjustment helps to ensure that the flow is easily reproducible for different ampul sizes.
Advertisement:
Tackling Attrition in Drug Discovery & Development
Please Join Aptuit at their Medicines Research Centre in Verona Italy
The Drug Discovery & Development process is an expensive, long and high-risk business taking more than 10 years. Often associated with a high attrition rate, it is driven by medical need, disease prevalence and the likelihood of success. On May 17th, 2011 Aptuit will welcome distinguished experts from academia and industry to this FREE symposium to discuss aspects and strategies to reduce attrition from target validation to clinical outcome. To learn more and to register, visit:  www.aptuit.com/verona/freesymposium www.aptuit.com/verona/freesymposium
|
|
|
|
 |
|
AlCana Technologies, the University of British Columbia, and Alnylam Pharmaceuticals have extended the companies' RNA interference (RNAi) therapeutics research collaboration for a third year. The research collaboration was initiated in August 2009 and focused on the discovery of cationic lipids used in lipid nanoparticles for the systemic delivery of RNAi therapeutics.

Amgen has expanded its operations in Brazil with the acquisition of Bergamo, a privately held Brazilian pharmaceutical company. Amgen also has agreed to reacquire rights in Brazil to its products that were previously granted to Mantecorp, a Brazilian pharmaceutical company.

The contract manufacturing organization AMRI has entered into a development and long-term exclusive commercial supply agreement with Parnell Manufacturing, an Australian company specializing in the research, development, and manufacture of veterinary products.
Advertisement:
Ropack, a leader in packaging solutions for the pharmaceutical, biopharmaceutical and nutraceutical industries, now provides the technology and expertise for stickpack packaging of solid oral dosage in a low relative humidity and temperature-controlled environment. Stickpacks – slim, tube-shaped packets the size of a stick of gum – offer impressive benefits: fill accuracy, portability, product differentiation, reduction in paper and foil usage, product differentiation and ideal sample option.
 www.ropack.com/en/service/primary-packaging/stick-pack www.ropack.com/en/service/primary-packaging/stick-pack
|
FDA has approved AstraZeneca's orphan drug vandetanib, a kinase inhibitor to treat medullary thyroid cancer that cannot be removed by surgery or that has spread to other parts of the body.
Advertisement:
Cleanroom Pass-Through Chambers - Terra Universal pass-throughs facilitate safe, clean parts transfer in and out of a cleanroom. High-reliability door interlocks prevent cross contamination by allowing only one door at a time to open. BioSafe™ Pass-Throughs feature coved corners and a double-wall, seamless design for easy sterilization. Roll-up doors, UV disinfection modules, HEPA air showers and a wide range of materials meet diverse requirements.
 http://terrauniversal.com/promotions/pt/pass-through-x-pt.php http://terrauniversal.com/promotions/pt/pass-through-x-pt.php
|
Camargo Pharmaceutical Services announced its expansion into Research Triangle Park with the opening of a new office in North Carolina.
The pharmaceutical and manufacturing company Cangene is making organization changes in its Canadian operations to reflect decreased US government contract-manufacturing activity and align its workforce with the company’s strategic focus on commercial products. The company is eliminating 40 positions and had previously eliminated 60 positions between Aug. 1, 2010 and Jan. 31, 2011, bringing the total positions eliminated in the current fiscal year to 100, or 12% of the company’s workforce. The company’s US contract-manufacturing operations, Cangene bioPharm, based in Baltimore, are not affected.
Advertisement:
Pharmaceutical starches from Grain Processing Corporation (GPC) range from basic unmodified starches for tableting to very specialized, innovative starches for unique applications. Spress® Pregelatinized Corn Starches NF perform as binders, disintegrants and lubricants for granulations, direct-compression tablets, capsules, dry blends and roller compaction. PURE-COTE® film-forming starches are designed for coating applications, oral thin films, capsules and any applications where clear, flexible films are required.
 Read more... Read more...
|
The biopharmaceutical company Cephalon said in a preliminary consent revocation statement filed with the US Securities and Exchange Commission (SEC) that its board of directors has recommended that shareholders reject the specialty pharmaceutical company Valeant Pharmaceuticals International's proposals to remove and replace Cephalon's current board of directors and not deliver any consent solicitation cards to Valeant. On Apr. 5, 2011, Cephalon's board rejected Valeant's unsolicited proposal to purchase the company for $73 per share, concluding that the nonbinding proposal is inadequate and not in the best interests of Cephalon's shareholders.
Advertisement:
GelJacket™ CO2 Incubator
Caron's new 10 cu. ft. GelJacket Benchtop IR-CO2 Incubator is the most innovative technological advancement for cell culture incubators in decades. GelJacket is active gel insulation, located in every incubator wall. GelJacket has thermal advantages far surpassing any other CO2 incubator. Units come standard with an IR sensor, controlled humidity, decontamination cycle and more! To learn more visit.
 www.caronproducts.com/geljacket www.caronproducts.com/geljacket
|
Compass Biotechnologies, a Edmonton-based company developing biosimilars and biobetters, has signed a memorandum of understanding (MOU) with PanGen Biotech, a South Korean company that develops Chinese hamster ovary cell lines. The terms of the MOU lay down the foundation for a definitive agreement to be signed within the next 60 days between the parties, whereby PanGen will manufacture under cGMP conditions, active pharmaceutical ingredients, which will be used to produce the biobetter versions of various drug products.
Advertisement:
The latest in news and information from Bosch Packaging Technology At our new Blog  http://boschpackagingpharmana.wordpress.com
Currently on the blog 150 years of Packaging History, single-use
dosing technology updates, and a capsule filling system comparison. http://boschpackagingpharmana.wordpress.com
Currently on the blog 150 years of Packaging History, single-use
dosing technology updates, and a capsule filling system comparison.
|
Eisai has established a new pharmaceutical sales subsidiary in Brazil, which will be named Eisai Participações Ltda. (Eisai Brazil). As a direct subsidiary of the company's US pharmaceutical operation Eisai Inc., Eisai Brazil will be based in San Paulo.
Advertisement:
Rapid delivery of accurate results with the superior sensitivity of Xevo TQ-S
The unprecedented levels of sensitivity, selectivity and accuracy of Xevo TQ-S from Waters, allows scientists to develop a fast flexible SPE/LC/MS/MS platform for the simultaneous quantitation of multiple amyloid ß peptides in cerebrospinal fluid for preclinical or biomarker discovery.  Learn more Learn more
|
Algeta, a company specializing in anticancer therapeutics, has formed a research collaboration with Genzyme to evaluate the potential of its Thorium platform. Under the terms of the collaboration, Genzyme will provide access to a proprietary tumor-targeting antibody, and Algeta will provide access to its thorium platform to attach the alpha-emitting payload thorium-227. Both companies will contribute resources toward the collaboration, which is expected to last for up to a year initially.
Advertisement:
Quality Tablet Press Replacement Parts Shipped Same Day!
By stocking over 300,000 replacement parts for nearly every press on the market, we're able to provide quality parts at incredible prices, faster than anyone else in the industry. All Natoli replacement parts are manufactured in the U.S.A. and are available for international delivery – and most items are available with same-day shipping!
Request a FREE QUOTE now!  http://www.natoli.com/parts http://www.natoli.com/parts
|
GlaxoSmithKline (GSK) has agreed to form a joint venture with the Medical Research Council, Imperial College of London, King's College of London, and the University College London that assumes responsibility for the facilities and operations at GSK's Clinical Imaging Center. Under the agreement, the center's operations and staff are expected to transfer to the newly formed joint venture in the third quarter of 2011. GSK has agreements with the joint venture to support its long-term engagement in drug discovery and imaging research.
Advertisement:
Management of Water/Cleaning Validation
Shimadzu's new TOC-L Series analyzers are ideal for the management of the total amount of organic carbon in pharmaceutical manufacturing processes. They feature the patented catalytic combustion oxidation method, fully automated sample pretreatment, and a wide measurement range, and when combined with a solid sample combustion unit, the carbon in attached residues can be measured for cleaning validation. Learn more Learn more
|
Millennium, the oncology arm of Takeda Pharmaceuticals, and the biopharmaceutical company Sunesis Pharmaceuticals have formed a license agreement for the development of Sunesis' oral, selective pan-Raf kinase inhibitor and one additional undisclosed kinase inhibitor program in oncology.
Advertisement:
Webcast Auction: May 10 at 11 AM ET.
Featuring processing & packaging equipment from a major pharmaceutical company! Lot catalog includes PK 'V' 100 Cubic Foot Blender, Elanco Shionogi Semi Automatic Capsule Filler, Kikusui Libra 36 Station Tablet Press, Marion 30 Cubic Foot Paddle Blender, AMF 340 qt Planetary Mixer and much more. Register & Bid Online at  EquipNet.com/Auctions. EquipNet.com/Auctions.
|
NextPharma, a contract development and manufacturing company, announced that it has enhanced its clinical trials services (CTS) facility in Göttingen, Germany through the installation of a ModuC LS IPC encapsulation/over-encapsulation capsule filling machine.
Novartis has completed its acquisition of the eye-care company Alcon. In other news, Novartis has licensed from GW Pharmaceuticals Sativex (delta-9-tetrahydrocannabinol and cannabidiol), an endocannabinoid modulator developed and manufactured by GW. The drug is designed to treat spasticity due to multiple sclerosis.
OctoPlus, a drug-delivery company, has signed a feasibility agreement with an undisclosed top-10 biopharmaceutical company to develop a controlled-release formulation.
Zacharon Pharmaceuticals, a company specializing in glycobiotechnology, has entered into a strategic research collaboration with Pfizer to develop drugs for orphan diseases, including lysosomal storage disorders. The potential value of the collaboration to Zacharon is approximately $210 million. The collaboration includes the potential development of compounds that may be discovered using Zacharon’s platform for developing small-molecule drugs targeting specific carbohydrate polymers or glycans.
Do you have news to report on facility expansions, contracts, service agreements, mergers, acquisitions, or personnel appointments? Send press releases to ptpress@advanstar.com |
|
|

|
Schott North America, a specialty glass and materials company, has appointed Linda S. Mayer as president and CEO, effective Apr. 1, 2011.
|
|
|
 |
|
|
FDA released a draft guidance for industry on Safety Labeling Changes—Implementation of Section 505(o)(4) of the Federal Food, Drug, and Cosmetic Act, on April 12. The draft guidance aims to put into practice requirements and enforcement tools called for by the 2007 FDA Amendments Act (FDAAA). Specifically, the Act authorizes FDA to require (or in some cases, order) certain drug and biological product application holders to make safety related labeling changes based upon new safety information that becomes available after approval of the product. The document applies to prescription drugs approved via NDA or ANDA and biological drugs approved via BLA.
Past practice involved the agency asking drug sponsors of approved products to make labeling changes related to safety after approval to address serious risks. The change was usually submitted in a supplement by the drug sponsor to FDA. The agency had little authority to enforce the changes, however, and negotiations between the drug sponsor and the agency were often needed, according to the draft guidance document. Now, under FDAAA, the agency can take action against a drug sponsor if requested labeling changes are not made. Enforcement actions include initiating proceedings to withdraw approval of the drug and notifying the public about safety information. The draft guidance details the definition of "new safety information," timeframes, and other components of such a labeling change. Comments are due by July 12, 2011.
FDA Commissioner Margaret Hamburg will testify before the US House Committee on Energy and Commerce, Subcommittee on Oversight and Investigations, on "Import Safety: Status of FDA's Screening Efforts at the Border" and Predictive Risk-based Evaluation for Dynamic import Compliance Targeting, on April 13.
FDA Center for Devices and Radiological Health Director Jeffrey Shuren is scheduled to testify before the US House Committee on Oversight & Government Reform, Subcommittee on Health Care, on the "Pathway to FDA Medical Device Approval: Is There a Better Way?" on April 14.
|
 |

|
Visit PharmTech.com/pharmtechtv to watch videos taped live during Interphex 2011 about the pharma industry in China, the state of continuous processing, and current challenges in outsourcing.

Coming Soon: In May, PharmTech.com will publish special content on bioprocessing and sterile manufacturing. |
|
|
|
 |
|
|
|
|

|
Immediate-Release Drug Delivery
What is driving new developments in immediate-release drug delivery?
|

View the poll archive. |
|
|
|
