|
|
 |


 |
|

|
Congress Overhauling US Patent Laws
The US patent laws are undergoing a major revision, the first large revision since the Patent Act of 1952. 
GlaxoSmithKline Plans to Divest Select OTC Brands
GlaxoSmithKline (GSK) identified certain over-the-counter (OTC) brands in its consumer healthcare business that the company plans to divest. 
USP Drug Quality Pilot Program Underway in Sub-Saharan Africa
On Apr. 18, 2011, scientists from the national laboratories of five African nations gathered in Accra, Ghana, to take part in a week of technical training put on by the US Pharmacopeia (USP) that will teach them how to detect substandard and counterfeit medicines. 
FDA and White House Combat Prescription Drug Abuse
On Tuesday, Apr. 19, 2011, officials from FDA, the US Department of Health and Human Services, and the Drug Enforcement Administration introduced President Obama’s plan for curbing prescription-drug diversion and abuse. 
|

|

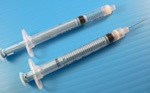 Prefilled syringes enhance safety Prefilled syringes enhance safety
Globe Medical Tech’s dual- and single-chamber prefilled syringes are designed to enhance safety. The syringes’ integrated needle-stick prevention feature and vacuum needle autoretraction technology provide full compliance with US and European safety regulations. After injection, the needle is retracted into the syringe body with a gentle push on the plunger. The syringe does not need any activation (e.g., twisting or capping) to engage the safety and contains no springs or metal components.
In combination with material and lubricant selections, the single- and dual-chamber syringes help maximize pharmaceutical compatibility. The dual-chamber syringe maintains the pharmaceutical components in separate chambers, thus extending the shelf life of some drugs. The syringes are made with medical-grade plastics that comply with FDA and international pharmacopoeial regulations. The plastics are compatible with a wide range of pharmaceutical drugs, and the components offer biocompatibility and low leachability. The syringes are designed to reduce dosing errors and contamination.
Advertisement:
Tackling Attrition in Drug Discovery & Development
Please Join Aptuit at their Medicines Research Centre in Verona Italy
The Drug Discovery & Development process is an expensive, long and high-risk business taking more than 10 years. Often associated with a high attrition rate, it is driven by medical need, disease prevalence and the likelihood of success. On May 17th, 2011 Aptuit will welcome distinguished experts from academia and industry to this FREE symposium to discuss aspects and strategies to reduce attrition from target validation to clinical outcome. To learn more and to register, visit:
 www.aptuit.com/verona/freesymposium www.aptuit.com/verona/freesymposium
|
|
|
|
 |
|
The biopharmaceutical company Access Pharmaceutical has formed an agreement with an undisclosed pharmaceutical company for its CobaCyte and CobOral technology for the targeted delivery of RNA interference (RNAi) therapeutics. Access will provide the pharmaceutical company with CobOral and CobaCyte siRNA formulations for evaluation of gene knockdown following oral and intravenous administration. Any successful formulation developed will be jointly owned by the parties and subject to a subsequent full licensing agreement.
Advertisement:
Ropack, a leader in packaging solutions for the pharmaceutical, biopharmaceutical and nutraceutical industries, now provides the technology and expertise for stickpack packaging of solid oral dosage in a low relative humidity and temperature-controlled environment. Stickpacks – slim, tube-shaped packets the size of a stick of gum – offer impressive benefits: fill accuracy, portability, product differentiation, reduction in paper and foil usage, product differentiation and ideal sample option.
 www.ropack.com/en/service/primary-packaging/stick-pack www.ropack.com/en/service/primary-packaging/stick-pack |
Axcan, a specialty pharmaceutical company, has agreed to acquire the biopharmaceutical company Mpex Pharmaceuticals. Mpex’s lead product candidate is Aeroquin, a proprietary aerosol formulation of levofloxacin that is currently in Phase III clinical trials for treating pulmonary infections in patients with cystic fibrosis. Financial terms were not disclosed. The deal is expected to close in the second half of 2011, subject to customary closing conditions.
Advertisement:
Management of Water/Cleaning Validation
Shimadzu’s new TOC-L Series analyzers are ideal for the management of the total amount of organic carbon in pharmaceutical manufacturing processes. They feature the patented catalytic combustion oxidation method, fully automated sample pretreatment, and a wide measurement range, and when combined with a solid sample combustion unit, the carbon in attached residues can be measured for cleaning validation.  Learn More Learn More |
Bend Research, a contract development and manufacturing organization, has received a US patent for improving bioavailability of low-solubility drugs. The patent, “Pharmaceutical Compositions Comprising Drug and Concentration-Enhancing Polymers” (US patent 7887840), was issued by the US Patent and Trademark Office in February. The technology covered by this patent does not require spray-drying or hot-melt extrusion formulation to improve bioavailability, but rather involves conventional blending of the drug form with a polymer additive. Drug forms covered by the patent are nanoparticles, absorbed drugs, drugs in nanosuspensions, supercooled drug melts, cyclodextrin/drug forms, gelatin dosage forms, and softgel dosage forms.
The custom manufacturing arm of Dr. Reddy’s Laboratories has expanded its Chirotech Technology Center at Cambridge Science Park, United Kingdom. The new 33,000-ft2 facility is designed for laboratories and offices and has been fitted to Dr. Reddy’s requirements for chemistry, biology, and analytics.
Advertisement:
Pharmaceutical starches from Grain Processing Corporation (GPC) range from basic unmodified starches for tableting to very specialized, innovative starches for unique applications. Spress® Pregelatinized Corn Starches NF perform as binders, disintegrants and lubricants for granulations, direct-compression tablets, capsules, dry blends and roller compaction. PURE-COTE® film-forming starches are designed for coating applications, oral thin films, capsules and any applications where clear, flexible films are required.
 Read More Read More
|
The specialty glass and materials company Gerresheimer has begun commercial production of ClikSTAR insulin pens for sanofi-aventis. The individual components of the ClikSTAR pens are manufactured at Gerresheimer’s Pfreimd, Germany, plant.
Advertisement:
GelJacket™ CO2 Incubator
Caron’s new 10 cu. ft. GelJacket Benchtop IR-CO2 Incubator is the most innovative technological advancement for cell culture incubators in decades. GelJacket is active gel insulation, located in every incubator wall. GelJacket has thermal advantages far surpassing any other CO2 incubator. Units come standard with an IR sensor, controlled humidity, decontamination cycle and more! To learn more visit.
 www.caronproducts.com/geljacket www.caronproducts.com/geljacket |
Johnson & Johnson (J&J) and Merck & Co. have agreed to amend the distribution rights to Remicade (infliximab) and Simponi (golimumab), which treat chronic inflammatory diseases such as rheumatoid arthritis. Under the agreement, Merck's subsidiary, Schering-Plough (Ireland) will relinquish exclusive marketing rights for Remicade and Simponi to J&J’s Janssen pharmaceutical companies in Canada, Central and South America, the Middle East, Africa and Asia Pacific, effective July 1, 2011. The retained territories by Merck include Europe, Russia, and Turkey.
Advertisement:
The latest in news and information from Bosch Packaging Technology At our new Blog
 http://boschpackagingpharmana.wordpress.com Currently on the blog 150 years of Packaging History, single-use
dosing technology updates, and a capsule filling system comparison. http://boschpackagingpharmana.wordpress.com Currently on the blog 150 years of Packaging History, single-use
dosing technology updates, and a capsule filling system comparison. |
In other news, the Ortho-McNeil Neurologics Division of Ortho-McNeil-Janssen Pharmaceuticals, announced this week that it is voluntarily recalling two lots of Topamax (topiramate) 100-mg tablets. These lots were shipped between Oct. 19, 2010 and Dec. 28, 2010 and distributed in the US and Puerto Rico. According to a company news release, there are probably fewer than 6000 bottles remaining in the marketplace. The recall is based on consumer reports of an uncharacteristic odor which may have been caused by TBA (2,4,6 tribromoanisole). This chemical has been associated with that used on the wooden pallets that are often used to store and transport packaging materials for medications.
Advertisement:
Rapid delivery of accurate results with the superior sensitivity of Xevo TQ-S
The unprecedented levels of sensitivity, selectivity and accuracy of Xevo TQ-S from Waters, allows scientists to develop a fast flexible SPE/LC/MS/MS platform for the simultaneous quantitation of multiple amyloid ß peptides in cerebrospinal fluid for preclinical or biomarker discovery.  Learn More Learn More |
Takeda Pharmaceutical has formed a two-year drug-discovery and development pact for G-protein coupled receptors (GPCR) with the biopharmaceutical company Heptares Therapeutics. Under the agreement, Takeda receives worldwide commercial rights to new drugs emerging from the collaboration. Takeda is making an upfront payment of £1.7 million ($2.8 million) to Heptares and is taking an equity stake of approximately £2.8 million ($4.6 million) in Heptares. Heptares is also eligible to receive future milestone payments of up to £60.5 million ($98.4 million) plus royalties on product sales.
Advertisement:
Quality Tablet Press Replacement Parts Shipped Same Day!
By stocking over 300,000 replacement parts for nearly every press on the market, we’re able to provide quality parts at incredible prices, faster than anyone else in the industry. All Natoli replacement parts are manufactured in the U.S.A. and are available for international delivery – and most items are available with same-day shipping!
Request a FREE QUOTE now!  http://www.natoli.com/parts http://www.natoli.com/parts |
The pharmaceutical company Vertex Pharmaceuticals and the Cystic Fibrosis Foundation Therapeutics have expanded their drug-development agreement in cystic fibrosis. The collaboration will support development activities for VX-661, Vertex's second corrector to enter clinical development, and the accelerated discovery and development of next-generation correctors. Vertex plans to begin the first study of VX-661 by the end of 2011 in people with cystic fibrosis who have the F508del mutation.
Advertisement:
Online Auction: May 24 at 9 AM ET.
Featuring processing & packaging equipment from Nature's Way (A Division of Schwabe North America). Lot catalog includes 60 Cubic Foot Marion Paddle Mixer, Manesty BB4 35 Station Tablet Press, Poly Pack Tray Former-Loader with Wrapper, Shrink Tunnel & Labeler, and much more. Register & Bid Online at  EquipNet.com/Auctions. EquipNet.com/Auctions. |
The generic-drug company Watson Pharmaceuticals has designated Parsippany, New Jersey, as the new site of its corporate headquarters (formerly located in Corona, California). The new 148,700-ft2 facility enables Watson to combine its operations, currently in separate facilities in the Morristown, New Jersey, area into a larger space.
West Pharmaceutical Services, a provider of systems, devices, and related services for injectable drugs, plans to increase capacity at its facility in Scottsdale, Arizona, for manufacturing the Daikyo Crystal Zenith 1-mL syringe system. The expansion will have the capacity of producing up to 20 million units annually. It will also handle the warehousing and release of other ready-to-use Crystal Zenith systems, such as vials and bulk drug containers. The expansion is scheduled to be completed in the fourth quarter of 2011.
Do you have news to report on facility expansions, contracts, service agreements, mergers, acquisitions, or personnel appointments? Send press releases to ptpress@advanstar.com |
|
|
|
|
|
|

|
Immediate-Release Drug Delivery
What is driving new developments in immediate-release drug delivery?
|

View the poll archive. |
|
|
|
