  |
 |
Bristol-Myers Squibb to Acquire ZymoGenetics
Bristol-Myers Squibb agreed to acquire the biopharmaceutical company ZymoGenetics for an aggregate purchase price of $885 million or $735 million net of cash. The move provides Bristol-Myers Squibb with additional biologic compounds for its drug pipeline. 
Lonza to Provide GSK with Biopharmaceutical Manufacturing
Last week, Lonza agreed to support the ongoing development of GlaxoSmithKline’s biopharmaceutical pipeline by supplying manufacturing capacity for five early-stage monoclonal antibodies. 
Actavis Signals Interest in Biosimilars Market
The generic-drug company Actavis is considering acquiring a 51% stake in the biopharmaceutical company BioPartners Holdings from Bioton, a Polish biotechnology company.
REACH Deadline Looming
The European Commission has issued a last call for registration in the Registration, Evaluation, Authorisation and restriction of Chemicals initiative. 

|

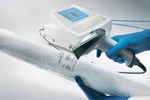 Instrument performs many filter-integrity tests Instrument performs many filter-integrity tests
The Sartocheck 4 plus instrument from Sartorius (Goettingen, Germany) performs a battery of filter-integrity tests such as diffusion, bubble point, diffusion–bubble point, water intrusion, water flow, customer-specific bubble point, customer-specific diffusion, and multipoint diffusion. The operator can select the test program manually or use a barcode reader to enter data into test programs. When the operator scans a filter’s barcode, the instrument recalls the appropriate test program for that filter automatically. The tester can connect to a network and exports results as PDF files that can be shared easily with other data systems.
In addition, operators can prompt the tester to clean itself automatically in about an hour. Users add cleaning solution into a separate vessel, and the tester cleans, rinses, and dries its entire pneumatic path. Operators thus do not need to send the instrument to the manufacturer for cleaning, and this feature reduces down time and cost.
|
|
|
 |
|
Acceleron Pharma (Cambridge, MA), a biopharmaceutical company developing therapeutics that modulate the growth of cells and tissues, and Shire (Dublin), a global specialty biopharmaceutical company, entered into a joint development and commercialization agreement for ACE-031 and other novel molecules targeting the activin receptor type IIB (ActRIIB) pathway. This pathway plays helps regulate the growth of skeletal muscle. Shire will receive an exclusive license to Acceleron’s ActRIIB molecules, including ACE-031, in markets outside of North America, and Acceleron will retain all commercial rights in North America. The collaboration will initially investigate ACE-031, Acceleron’s lead ActRIIB drug candidate, currently in a Phase II a trial for the treatment of patients with Duchenne muscular dystrophy.
Anchor Therapeutics (Cambridge, MA) entered into a collaboration and license agreement with Ortho-McNeil-Janssen Pharmaceuticals (OMJPI, New Brunswick, NJ) with the goal of developing G protein coupled receptor (GPCR)-targeted therapeutic compounds using Anchor’s proprietary pepducin technology. Under the agreement, Anchor and OMJPI will work jointly to discover and optimize preclinical development candidates against GPCR targets in oncology and metabolic disorders, including Anchor’s program targeting gpr39, a GPCR involved in metabolic diseases. OMJPI will assume responsibility for development and commercialization. Anchor will receive an up-front payment and research support and could be eligible for development and regulatory milestone payments of as much as $480 million.

AstraZeneca (London) and University College London entered into a collaboration to develop regenerative medicines for diabetic retinopathy. Under the terms of the three-year agreement, AstraZeneca and scientists at the UCL Institute of Ophthalmology will collaborate to identify new therapeutic tools that can modulate the regenerative capacity of stem cells. Diabetic retinopathy is now the most common cause of vision impairment among people of working age in Western countries.
Advertisement:
Comply with ICH guidelines on impurities in new drug products!
The Agilent 2100 Bioanalyzer plus High Sensitivity Protein 250 kit automatically detects and quantifies protein impurities down to 0.05% - meeting ICH guideline Q3B(R2)!
 Request Agilentís Protein QC Application CD! Request Agilentís Protein QC Application CD!
|
Bend Research (Bend, OR), a drug-formulation development and manufacturing company, is expanding its pharmaceutical manufacturing capabilities for hot-melt extrusion. The company purchased intermediate-scale equipment for hot-melt extrusion at its contract manufacturing facility to provide additional processing options for its clients. The new equipment, an 18-mm extruder, provides capacity between existing 7.5- and 27-mm extruders. This extruder enables manufacture at development scale.
Celldex Therapeutics (Needham, MA) will regain full worldwide rights to develop and commercialize rindopepimut (CDX-110), effective November 1, 2010. Celldex and Pfizer Vaccines (New York) entered into a global development and commercialization agreement in April 2008 for rindopepimut, an experimental therapeutic cancer vaccine that targets the tumor-specific molecule epidermal growth factor receptor variant III (EGFRvIII) in patients with glioblastoma multiforme. Pfizer informed Celldex that the rindopepimut program is no longer a strategic priority and has terminated the agreement.
Advertisement:
Shimadzu
Powerful, Easy-to-Use X-ray Diffractometers Shimadzu's X-ray Diffractometers (XRD) feature a polycapillary parallel-beam optics system, which allows highly sensitive and accurate measurements of samples with curved or irregular surfaces. In addition, with fast measurements, high stability, easy operation, and advanced control, analysis and reporting software, our XRDs enable routine identification and quantification of a number of samples - even for beginners.
Learn more at
 www.ssi.shimadzu.com/MT www.ssi.shimadzu.com/MT
|
The US Court of Appeals for the Second Circuit agreed with Eli Lilly and Company’s (Indianapolis) position that a class should not have been certified in a pending third-party payor suit, in which unions and insurers who act as third-party payors alleged that they overpaid for Zyprexa prescriptions. The court also agreed with Lilly that plaintiffs’ overpricing claims should not go forward.
Genzyme (Cambridge, MA) entered into an asset-purchase agreement under which Laboratory Corporation of America Holdings (LabCorp, Burlington, NC) will acquire Genzyme Genetics for $925 million in cash. LabCorp will purchase the business in its entirety, including all testing services, technology, intellectual property rights, and its nine testing laboratories. The agreement is subject to customary closing conditions, including the Hart-Scott-Rodino Antitrust Improvements Act of 1976, and is expected to close before the end of the year.
Jennerex (San Francisco, CA), a private clinical-stage biotherapeutics company, and Transgene (Illkirch Graffenstaden, France), a biopharmaceutical company, entered into an exclusive partnership to develop and commercialize JX-594 for the treatment of solid tumors in Europe, the Commonwealth of Independent States, and the Middle East. JX-594, Jennerex’s lead cancer biotherapeutic product, has shown anticancer activity and a well-tolerated safety profile in Phase I and Phase II clinical trials to date.
Advertisement:
CBI
3rd West Coast Clinical Supply Chain Management - November 16-17, San Francisco, CA Regulatory Guidance, Operational Efficiencies and Proven Strategies for Global Supply Chain Excellence. A must-attend event for supply chain and logistics professionals! Network with peers and discuss emerging trends, best practices and lessons learned. Industry leaders from Allergan, Centocor, Cerexa, Merck, Santen, Zosana and more!
Register at
 www.cbinet.com/supplychainwest
and save $400 with code DAN757! www.cbinet.com/supplychainwest
and save $400 with code DAN757! |
K-V Pharmaceutical’s (St. Louis, MO) facilities successfully completed a US Food and Drug Administration inspection, and the agency allowed the company to return its first product to market. Under the company’s consent decree, successful FDA inspections of the company’s quality systems, processes, and facilities are expected to be required before K-V is permitted to resume manufacturing and shipment of particular products.
Mylan (Canonsburg, PA) completed the acquisition of Bioniche Pharma Holdings Limited (Galway, Ireland) sooner than anticipated. Bioniche Pharma, an injectable pharmaceutical company, is providing Mylan with immediate entry into the North American injectables market and a platform for the commercialization of future biosimilar product offerings. Mylan acquired Bioniche Pharma for $550 million in cash and expects the business to be accretive in year one, without accounting for any synergies.
Santarus (San Diego, CA), a specialty biopharmaceutical company, expanded its development pipeline with the addition of two biologic drug candidates focused on specialty markets. Santarus signed exclusive license and supply agreements with Pharming Group (Leiden, The Netherlands) granting Santarus the right to commercialize RHUCIN (recombinant human C1 inhibitor) in North America for the treatment of acute attacks of hereditary angioedema and other future indications. Santarus also acquired the worldwide rights to a biologic drug candidate, an anti-VLA-1 antibody that has shown activity in multiple preclinical models of inflammatory and autoimmune diseases, through the acquisition of Covella Pharmaceuticals, and by amending a related license agreement with Biogen Idec (Weston, MA).
Vyteris (Fair Lawn, NJ) a drug-delivery technology company, executed a definitive merger agreement with MediSync BioServices, a privately held company focused on the consolidation of complementary, niche contract research organizations, site-management organizations, and related businesses within the contract drug-development industry.
Do you have news to report on facility expansions, contracts, service agreements, mergers, acquisitions, or personnel appointments? Send press releases to ptpress@advanstar.com |
|
|
 |

|
AAIPharma Services (Wilmington, NC), a provider of pharmaceutical product-development services, appointed Patrick D. Walsh as its chief executive officer. Walsh will focus on advancing AAIPharma’s next phase of strategic growth, following its establishment as an independent company. Walsh has served in executive roles at several pharmaceutical companies, including as president and CEO of Kadmus Pharmaceuticals (Irvine, CA), and president and COO of Gensia Sicor (Irvine, CA).
AVEO Pharmaceuticals (Cambridge, MA), a biopharmaceutical company focused on discovering, developing, and commercializing cancer therapeutics, appointed Michael P. Bailey to the newly created role of chief commercial officer. The company also named Donna M. Radzik its vice-president of technical operations. Both positions will report to Elan Ezickson, executive vice-president and chief business officer of AVEO.
Bausch and Lomb (Rochester, NY), the global eye-health company, named Daniel M. Wechsler corporate vice-president and global president of its pharmaceuticals business. Wechsler was most recently head of US strategy, commercial model innovation, and business development for Merck & Co. (Whitehouse Station, NJ), a role to which he was appointed following the company's acquisition of Schering-Plough in 2009.
bluebird bio (Cambridge, MA), a developer of gene therapies for severe genetic disorders, appointed Nick Leschly the company’s president and chief executive officer. Leschly formerly was the company’s interim president and partner of Third Rock Ventures.
ChanTest (Cleveland, OH) named D. Thomas Oakley president and chief executive officer. Oakley most recently served as president and chief executive officer of Bridge Pharmaceuticals (Gaithersburg, MD), a global preclinical contract research organization with operations in the US and China.
Brian Koski joined Chemsultants International (Mentor, OH) as logistics manager for the company’s product- and process-development business unit. Koski will report to Tom Besselman, the company’s director of operations. Koski will manage the overall materials supply stream of Chemsultants pilot coating, new product scale-up, and specialty contract-manufacturing operations. He will provide planning, oversight, and direction for materials purchasing, sourcing, supplier relations, inventory management, and warehousing activities.
William H. Baum joined Genomatica (San Diego, CA) full time as the company’s executive chairman and chief business-development officer. Baum will oversee Genomatica’s strategic partnerships with leading chemical producers, users, feedstock suppliers, brands, and retailers.
SARcode (San Francisco), a biotechnology company developing a class of lymphocyte function-associated antigen-1 (LFA-1) antagonists, hired Quinton Oswald as chief executive officer. Oswald most recently served as the vice-president and business-unit head for the tissue-growth and -repair business at Genentech (South San Francisco, CA). |

|
The International Society for Pharmaceutical Engineering (ISPE) will host the ISPE Risk-MaPP 2010 Conference, Dedicated Facilities, Cross Contamination and the Risk-MaPP Approach in Washington, DC, on October 4–5 2010. The group will hold the North American launch of the new ISPE Baseline Guide: Risk-Based Manufacturing of Pharmaceutical Products (Risk-MaPP) at the conference, and attendees will receive a complimentary downloadable copy of the guide. To register for the conference, click here. |
|
|
|
|
 |
|
|
