|
|
 |

|
 |
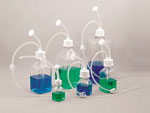 Tube and cap system for handling biologicals Tube and cap system for handling biologicals
The SaniSure Cap2v8 system is an integrated cap and tube system that provides aseptic transfer, storage, and sampling applications. Its one-piece molded cap reduces contamination and product loss that traditionally is associated with barbed fittings.
The flexibility of this product line allows customers the ability to custom configure systems with unique tubing sizes, lengths, and other accessories. The standard Cap2v8 components use C-Flex tubing, and the caps are made of polypropylene (other materials are available).
The SaniSure Cap2v8 system is designed for single-use, meets USP Class VI standards, is lot-traceable, is packaged in a certified ISO-7 cleanroom, and provides quick turnaround lead times on small or large-volume lots. With the option to have these components made into complete assemblies and shipped presterilized or not presterilized, customers have the flexibility of implementing technology specific to their specific needs.
|
|
|
 |
|
Alnylam Pharmaceuticals and Tekmira Pharmaceuticals have restructured their relationship with a new licensing agreement and have resolved all litigation between the parties in a settlement agreement. The new license agreement consolidates and clarifies certain intellectual property elements related to lipid nanoparticle (LNP) technology for RNAi therapeutics. Further, Alnylam has elected to independently manufacture its LNP-based RNAi therapeutic products and to buy-down certain future potential milestone payments and a significant portion of future potential royalties for its ALN-VSP, ALN-PCS, and ALN-TTR02 programs. Read More
Advertisement:
VitalDose® Excipients for Customizable Delivery
Learn more about VitalDose® EVA excipients on the VitalDose website and blog. Learn how EVA polymers have been used for years in controlled release pharmaceutical and medical device applications. VitalDose EVA can assist in the tailored dosing and delivery of your API and offers manufacturing process versatility and multiple routes of administration. VitalDose@Celanese.com or  www.VitalDose.com/blog. www.VitalDose.com/blog. |
GlaxoSmithKline (GSK) has reached an agreement with XenoPort to terminate their collaboration concerning Horizant (gabapentin enacarbil) extended-release tablets, for which GSK had commercialization rights and certain development rights in the US. Under the termination and transition agreement, GSK is returning Horizant rights to XenoPort and providing certain assistance during the transition period ending Apr. 30, 2013, upon mutually agreed terms. The decision to return the asset is aligned with GSK’s ongoing strategy to streamline its portfolio to focus on core franchise opportunities. The agreement also resolves all litigation between the parties. XenoPort acknowledges that GSK fulfilled its contractual obligations on the development, manufacturing, and commercialization of Horizant. Read More
Advertisement:
Free Technical Posters for Tablet Manufacturers
The latest edition to Natoli's extensive collection of free technical resources, the Technical Poster Series features 3 large-format posters packed with helpful tips, charts, terminology, and more. For a limited time, all 3 posters are available at no charge shipping included so order yours now!  http://www.natoli.com/Posters-PT.html http://www.natoli.com/Posters-PT.html
|
Neptune Technologies & Bioressources, a manufacturer of phospholipid products for the nutraceutical and pharmaceutical industries, reported that, on Nov. 8, 2012, an explosion and fire destroyed its production plant located in Sherbrooke, Quebec, Canada. Three employees were fatally injured. Eighteen other people were transported to the hospital—four of whom were severely injured. The incident completely destroyed Neptune’s current production plant that was in operation in Sherbrooke, but damages at the expansion facility currently under construction adjacent to Neptune’s Sherbrooke plant appear to be limited, according to the company. Neptune is strategizing on an action plan going forward to allow it to resume production and meet client demands, and plan particulars will be announced at a later date.
A roundup of additional company and people news from pharmaceutical and biopharmaceutical companies, their suppliers, and contract-service providers. Read More
Advertisement:
Vindon Scientific (USA) provide outsourced storage to clients in our stability storage suite in Atlanta, encompassing walk in rooms and chambers, a complete range of World Climatic ICH conditions as well as unique conditions.
In addition Vindon Scientific manufacture and distribute stability storage walk in rooms, reach in rooms and chambers for the pharmaceutical and chemical industry.  www.vindonscientific.com www.vindonscientific.com
|
Do you have news to report on facility expansions, contracts, service agreements, mergers, acquisitions, or personnel appointments? Send press releases to ptpress@advanstar.com |
|
|
 |
|
|
Encouraging Orphan Drugs
by Jill Wechsler
Pharmaceutical companies, scientists, and regulators are championing the development of new treatments for rare diseases as key to spurring biomedical discovery. Experts at an October conference on rare diseases sponsored by the National Organization for Rare Disorders and the Drug Information Association discussed FDASIA provisions that expand FDA staff, guidance, and public outreach to support orphan drug research and approval. John Jenkins, director of the Center for Drug Evaluation and Research (CDER) Office of New Drugs, outlined additional FDASIA policies that provide assistance to sponsors of "breakthrough therapies," expand accelerated approval of drugs to treat life-threatening conditions, and special incentives for developing treatments for rare pediatric conditions.
Advertisement:
 Catalogs of Innovative Lab and Clean Room Equipment Catalogs of Innovative Lab and Clean Room Equipment
– technical resources and competitive pricing on popular lines of laboratory products from Terra Universal. Streaming eCatalogs contain application tutorials and instructional video on controlled-temperature systems, sample preparation equipment, and analytical instrumentation. Terra also manufactures complete lines of critical environment enclosures, including hoods, isolation chambers, desiccators, modular clean rooms and pass-through chambers. |
Beyond FDA, the National Center for Advancing Translational Sciences at the National Institutes of Health is supporting development of therapeutics for rare and neglected diseases through its drug "repurposing" program plus collaborative research projects. Venture capitalists, moreover, eye more flexible clinical research requirements for orphan drugs as a way for biopharmaceutical companies to gain regulatory approval more quickly and efficiently.
FDA Updates
FDA has provided information on its Enhanced Communication Team created in response to PDUFA V and FDA's promotion of innovation through interactive communication between the agency and sponsors during drug development.
The following industry guidance documents have been released by FDA:
Generic drug-user fee notice, October 2012: manufacturers will pay approximately $17,500 for FDA's Office of Generic Drugs to process a backlogged abbreviated new drug application (ANDA). The first manufacturer to reference a drug master file will pay a one-time fee of $21,340. Filing an ANDA will cost $51,520 at time of submission, and about half that amount for a prior approval supplement. Facility fees are due from FDA by mid-January and will be higher for foreign manufacturers.
|
|
 |

| Optimizing Early-Stage Drug Development |
by: Patricia Van Arnum
Pharmaceutical companies and contract service providers adapt strategies and capabilities to reduce costs and accelerate drug-development timelines.
 |
Coming soon: Pharm Tech's December issue will examine the new small-molecule and biologic-based drugs to receive FDA approval in 2012 and offers an outlook for promising late-stage candidates. |
|
|
Know Before You Go2012 Conference Previews
Visit our Show & Exhibition Gateway 2012 to see which companies are exhibiting at upcoming industry conferences.
Find company backgrounds, new product releases, booth materials, and more.
PharmTech's online
Gateway is your guide to the season's leading shows.
|
|
|
|
|
|
