Is FDA's Draft Process-Validation
Guidance a Mixed Blessing?
The US Food and Drug Administration’s draft Guidance for Industry—Process Validation: General Principles and Practices includes several concepts that are familiar to the industry. It also contains ambiguities and recommendations that might be difficult for some drugmakers to follow.
 |
Reducing Energy and Water Use in Process Cooling
Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

>>Coming Soon: To find out how robots could aid pharmaceutical manufacturing, see the cover story of the September issue of Pharmaceutical Technology.
|

AEC (Schaumburg, IL) offers the 10/80 unit, the latest addition to its VacTrac series of conveying-control equipment. |
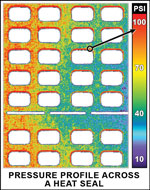
Sensor Products (Madison, NJ) introduced its Pressurex pressure-indicating sensor film.  |

 |
 |
- NewAge Industries (Southampton, PA) offers Suprene thermoplastic rubber tubing in US Food and Drug Administration and Industrial grades.
- Honeywell Process Solutions (Phoenix, AZ) has introduced the FlexLine wireless radar gauge, which helps process manufacturers monitor tank levels and prevent hazardous incidents in their plants and terminals.
- Pilgrim Software (Tampa, FL) offers its SmartInsight Report Writer, which allows users to develop, format, and control the content of reports and distribute them to the right users.
- ARmark Authentication Technologies (Glen Rock, PA) and Smiths Detection (Watford, England) have launched the IntelliMark system for verifying genuine brand and product authentication for consumer goods and secure documents.
- Spiroflow Systems (Monroe, NC) provides its PNEUVAC conveying systems for products that raise degradation concerns.
|
 |
-
Sep. 16–17: INTERPHEX Canada (Toronto). Attendees of INTERPHEX Canada will view the latest technological innovations from world-class exhibitors, network with peers in the pharmaceutical, biotech, and life-sciences industries, and keep pace with the constantly evolving marketplace.
-
Sep. 21–24: Process Excellence Week (Chicago). How safe do you think your business is from falling profits in the current economic climate? Want to learn how to protect it by using the latest, most relevant Process Excellence tools around today? This fast-paced 5th Annual conference delivers the newest, most powerful methods and quicker-to-implement projects for delivering significant bottom line results for your entire organization.
-
Sep. 23–24: Packaging Materials for Packaging Professionals (Philadelphia). This seminar gives packaging-industry professionals a sound understanding of the science and applications of various packaging materials. An understanding of the underlying principles of materials science is beneficial when troubleshooting packaging problems and developing packaging. This two-day seminar covers fundamental concepts of materials science and applies them to common packaging materials such as plastics, metals, papers, and composites.
-
Sep. 24–25: Advanced Conducting Bulletproof CAPA Investigations Course (Philadelphia). Class topics include US Food and Drug Administration regulatory requirements and expectations, how FDA inspects corrective and preventive actions (CAPA) systems and investigations, managing CAPA, best practices, how to conduct an investigation, root cause analysis, report writing, required FDA notifications, and risk management as it applies to CAPA.
-
Sep. 30–Oct. 2: EUDRAGIT 3-Day Workshop: Hot Melt Extrusion and Modified-Release Applications (Piscataway, NJ). This three-day workshop will provide a detailed understanding of the chemistry and application technologies of EUDRAGIT polymers in pharmaceutical applications. It will include numerous in-depth presentations on gastrointestinal targeting, modified release, taste masking, and solubility enhancement. Lectures will be supplemented by demonstrations with state-of-the-art equipment in a process-development laboratory.
|
|
|
| PharmTech
Poll: |
>>FDA’s Enforcement Plan
FDA recently outlined six steps the agency will take to increase enforcement of its regulations. What do you think of the agency’s plan? |
|
 |
|
|
|
|