
An Unfocused Handbook on Environmental Monitoring
An expert-panel-written book has surprising shortcomings.

>>Coming Soon: For a guide to the considerations involved in buying used packaging machinery, look for Hallie Forcinio’s “Packaging Forum” column in the November issue of Pharmaceutical Technology.
|

EMD Millipore’s (Billerica, MA) Enhanced Mobius 3L CellReady single-use bioreactor includes additional features intended to enhance its operational flexibility.
 |
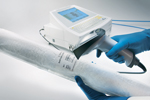
The Sartocheck 4 plus instrument from Sartorius (Goettingen, Germany) performs a battery of filter-integrity tests such as diffusion, bubble point, diffusion–bubble point, water intrusion, water flow, customer-specific bubble point, customer-specific diffusion, and multipoint diffusion.
 |
 |
Kavon Filters
Fluid-bed dryer bags Kavon provides custom replacement fluid-bed dryer bags for US and European equipment models. The bags are appropriate for wet granulation, dry filtration, and wet and dry coating applications. The company offers flexible 1-4-bag systems in various fabrics choices and also repairs bags. Kavon Filter Products, Kavon Filter Products, Wall Township, NJ | www.kavonfilter.com | tel. 732.938.3135

|
|
| |
The “NEW” state-of-the-art Top Line diaphragm valve actuator combines exceptionally convenient features with full engineering support to provide you with a TOTAL automation solution. There’s currently no actuator in the sanitary market that can match the outstanding features and benefits of this actuator. You can maintain and change the function of the actuator head without opening the sterile pipeline. The actuator is “The ONLY one of its kind!”

|
|
| |
Veriteq Instruments
Vaisala / Veriteq Instruments (image attached) How to Respond to (and Avoid) FDA 483s Learn the ten best practices for formulating an effective 483 response. This article shows common observations and provides checklist to make the15-day response time limit more manageable, methods for improving quality systems for controlled environments, and links for further reading and reference. Read the entire article at www.veriteq.com/contact/fda_483_paper.htm

|
|
| |
|
Pure Sealing Systems
BioClosure™ Systems offer the utmost in purity for sealing glass, metal, or plastic containers. Manufactured from USP Class VI, platinum-cured silicone in a Class 7, ISO-certified clean room.
AdvantaPure®
http://www.advantapure.com/bioclosure-container-closures.htm

|
|
| |
|
ChemInstruments Adhesive Testing Equipment
ChemInstruments manufactures a complete line of testing equipment to measure the properties of peel adhesion, adhesive tack, adhesive shear strength plus material properties such as coefficient of friction, and tensile strength. www.cheminstruments.com

|
 |
| |
|
Midwesco's Pleat+Plus® filter
Midwesco's Pleat+Plus® filter elements can increase the filtration surface area in your baghouse system by significantly reducing the air-to-cloth ratios.

|
 |
| |
|
The Palltronic® Flowstar IV filter integrity test instrument is fast, accurate and designed for use in laboratories or cleanrooms. Offering the latest networking and automation options, it is supported by extensive qualification documentation with worldwide technical and aftersales support. For more information, please email flowstar@pall.com
|
 |
| |
|
Free Webinar: Ben Miyares presents “The Future of Induction Sealing”
Join Moderator Ben Miyares and experts from the packaging field including Selig and Enercon for this free event. Find out how recent packager focused developments are improving the reliability, performance, communications and operation of induction sealing. Panelists will discuss MES/EMI data network integration, operator interface, sustainable materials, anti-counterfeit solutions and much more.

|
 |
|
 |
- Acsis’s (Marlton, NJ) Serialized Packaging Data Management module, a new component of its ProducTrak for Life Sciences software, enables bidirectional data transfer between packaging lines and an enterprise resource planning system.
- Cole-Parmer (Vernon Hills, IL) has greatly expanded its line of filtration products from Advantec.
- Meissner’s (Camarillo, CA) TepoFlex single-use biocontainers are designed to provide secure, robust fluid-handling options.
- The HygroPalm23 portable water-activity set from Rotronic (Hauppauge, NY) is designed to aid the quality inspection of humidity-sensitive products.
- Thermo Fisher Scientific (Waltham, MA) has introduced the La-Pha-Pack stainless-steel cleanroom crimping tools for a controlled, low-effort method of vial sealing.
|
 |
- Oct. 27–29: European Conference on Active Pharmaceutical Ingredients (Barcelona). At this conference, speakers from the US Food and Drug Administration, the European Commission, the World Health Organization, the US Pharmacopeia, and industry will discuss the latest developments in good manufacturing practice and regulatory compliance.
- Oct. 31–Nov. 3: Pack Expo International 2010 (Chicago). This show presents an array of packaging and processing innovations on one of the industry’s most dynamic show floors. Attendees will learn about the trends, technologies, and integrated solutions that help optimize operations across the line.
- Nov. 3–5: Fifth Annual FDA Inspections Summit (Bethesda, MD). The Annual FDA Inspections Summit is one of the premier annual regulatory inspections events in the US. The conference regularly brings together top industry leaders.
- Nov. 5: Corrective and Preventive Actions (Farmingdale, NY). This conference, hosted by the American Society for Quality, will feature speakers from the US Food and Drug Administration, Sparta Systems, and Northrup Grumman.
- Nov. 16–18: Risk-Based Decision Making (Burlingame, CA). This course will focus on good decision making and the appropriate application of risk-based decision making tools and techniques. The event is geared toward people working in quality, manufacturing, product and process development, and validation.
|
|
|
| PharmTech
Poll: Cleanroom Challenges |
What is the biggest challenge to implementing cleanroom conditions? |
|
 |
|
|
| |

|
|
|
|
|