| ALECENSA® (alectinib) Provides Breakthrough PFS With CNS Efficacy in 1L ALK+ mNSCLC1 |
| The efficacy and safety of ALECENSA were established in the head-to-head, global, open-label, Phase 3 ALEX trial that randomized 303 patients with stage IIIB/IV ALK+ mNSCLC to receive ALECENSA 600 mg orally twice daily or crizotinib 250 mg orally twice daily. Treatment on both arms continued until disease progression or unacceptable toxicity. The primary efficacy endpoint was PFS as determined by Investigator based on RECIST v1.1. Additional efficacy endpoints included PFS (based on RECIST v1.1), ORR, DOR, and time to cause-specific CNS progression as determined by IRC and OS as determined by Investigator.1,2 |
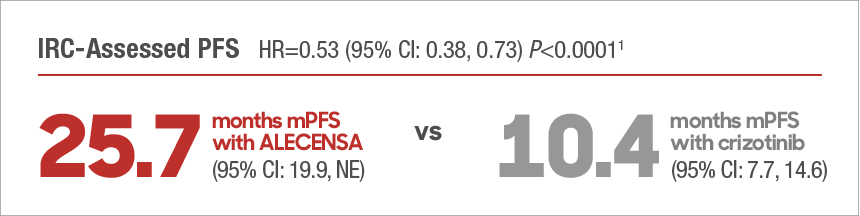
| ALECENSA Significantly Improved PFS vs Crizotinib |
 |
|
|
 |
| • |
Median duration of follow-up: 18.6 months (range: 0.5-29.0 months) for ALECENSA and 17.6 months (range: 0.3-27.0 months) for crizotinib2 |
| • |
Investigator-assessed mPFS was not estimable (HR=0.48 [95% CI: 0.35, 0.66]; P<0.0001)1 |
|
 |
|
|
 |
| The follow-up analysis was performed when approximately 50% of patients in the ALECENSA arm experienced a PFS event and was conducted for the purpose of obtaining a median estimate of PFS. No formal treatment comparisons were performed for the follow-up analysis.3 |
| • |
Median duration of follow-up: 27.8 months (range: 0.5-38.7 months) for ALECENSA and 22.8 months (range: 0.3-36.7 months) for crizotinib3,4 |
| ◦ |
There was no additional IRC assessment performed at this time4 |
|
|
| Select Important Safety Information |
| Hepatotoxicity |
| • |
Of 405 patients, elevations of AST >5X the upper limit of normal (ULN) occurred in 4.6% of patients, and elevations of ALT >5X the ULN occurred in 5.3% of patients. Elevations of bilirubin >3X the ULN occurred in 3.7% of patients. Six patients discontinued ALECENSA for Grades 3-4 AST and/or ALT elevations, and 4 patients discontinued ALECENSA for Grade 3 bilirubin elevations. Three patients with Grades 3-4 AST/ALT elevations had drug-induced liver injury |
| • |
Monitor liver function tests every 2 weeks during the first 3 months of treatment, then once a month and as clinically indicated, with more frequent testing in patients who develop transaminase and bilirubin elevations. Based on the severity of the adverse drug reaction, withhold then dose reduce, or permanently discontinue ALECENSA |
|
| Learn More About Breakthrough 1L PFS |
 |
|
|
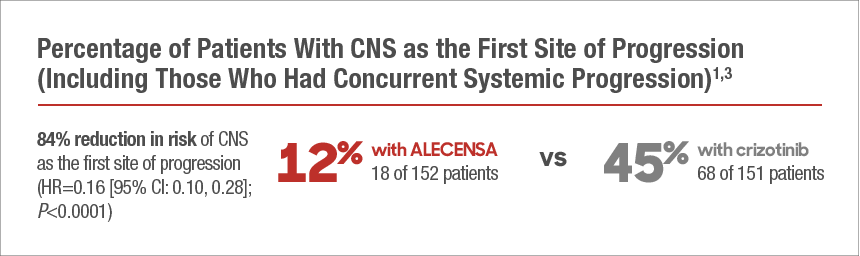
| ALECENSA Significantly Reduced the Risk of CNS as the First Site of Progression |
 |
|
|
| Time to cause-specific CNS progression was defined as CNS as the first site of progression alone or with concurrent systemic progression. Patients who first progressed systemically, and patients with death prior to CNS or systemic progression were not included as events.1,3 Cause-specific HR and 95% CI were estimated by Cox model where patients with competing events (systemic progression and death prior to CNS or systemic progression) were censored at the time of these events. P-values were estimated from two-sided stratified cause-specific log-rank tests.5 |
| Select Important Safety Information |
| The Warnings and Precautions for ALECENSA include: |
|
| • |
Hepatotoxicity |
| • |
ILD/pneumonitis |
| • |
Renal impairment |
| • |
Bradycardia |
| • |
Severe myalgia and CPK elevation |
| • |
Embryo-fetal toxicity |
|
| Please see additional Important Safety Information in full Prescribing Information. |
| Discover More About ALECENSA |
|
|
|
|
|
|
|
|
|
|
| 1L=first-line; CI=confidence interval; CNS=central nervous system; DOR=duration of response; HR=hazard ratio; IRC=Independent Review Committee; mPFS=median progression-free survival; NE=not estimable; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumors. |
| References: 1. ALECENSA [prescribing information]. South San Francisco, CA: Genentech USA, Inc; 2018. 2. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017; 377:829-838. 3. Data on file. Genentech, Inc. 4. Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (AL) versus crizotinib (CZ) in untreated advanced ALK+ NSCLC. Poster presented at: 2018 American Society of Clinical Oncology Annual Meeting; June 1-5, 2018; Chicago, IL. 5. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377(suppl):1-15. |
|
|
| Contact a Representative |
| Indication |
|
| ALECENSA is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (mNSCLC) as detected by an FDA-approved test. |
| Important Safety Information |
|
| Warnings and Precautions |
| Hepatotoxicity |
| • |
Of 405 patients, elevations of AST >5X the upper limit of normal (ULN) occurred in 4.6% of patients, and elevations of ALT >5X the ULN occurred in 5.3% of patients. Elevations of bilirubin >3X the ULN occurred in 3.7% of patients. The majority (69% of the patients with hepatic transaminase elevations and 68% of the patients with bilirubin elevations) of these events occurred during the first 3 months of treatment. Six patients discontinued ALECENSA for Grades 3-4 AST and/or ALT elevations, and 4 patients discontinued ALECENSA for Grade 3 bilirubin elevations. Concurrent elevations in ALT or AST ≥3X the ULN and total bilirubin ≥2X the ULN, with normal alkaline phosphatase, occurred in <1% of patients treated with ALECENSA across clinical trials. Three patients with Grades 3-4 AST/ALT elevations had drug-induced liver injury |
| • |
Monitor liver function tests including ALT, AST, and total bilirubin every 2 weeks during the first 3 months of treatment, then once a month and as clinically indicated, with more frequent testing in patients who develop transaminase and bilirubin elevations. Based on the severity of the adverse drug reaction, withhold ALECENSA and resume at a reduced dose, or permanently discontinue ALECENSA |
|
| Interstitial Lung Disease (ILD)/Pneumonitis |
| • |
ILD/pneumonitis occurred in 3 (0.7%) patients treated with ALECENSA. One (0.2%) of these events was severe (Grade 3) |
| • |
Promptly investigate for ILD/pneumonitis in any patient who presents with worsening of respiratory symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, and fever) |
| • |
Immediately withhold ALECENSA treatment in patients diagnosed with ILD/pneumonitis and permanently discontinue ALECENSA if no other potential causes of ILD/pneumonitis have been identified |
|
| Renal Impairment |
| • |
Renal impairment occurred in 8% of patients. The incidence of Grade ≥3 renal impairment was 1.7%, of which 0.5% were fatal events |
| • |
Dose modifications for renal impairment were required in 3.2% of patients. Median time to Grade ≥3 renal impairment was 3.7 months (range 0.5 to 14.7 months) |
| • |
Permanently discontinue ALECENSA for Grade 4 renal toxicity. Withhold ALECENSA for Grade 3 renal toxicity, then resume at reduced dose |
|
| Bradycardia |
| • |
Cases of bradycardia (8.6%) have been reported in patients treated with ALECENSA. Eighteen percent of 365 patients treated with ALECENSA for whom serial ECGs were available had heart rates of <50 beats per minute (bpm) |
| • |
Monitor heart rate and blood pressure regularly |
| • |
In cases of symptomatic bradycardia that are not life-threatening, withhold ALECENSA until recovery to asymptomatic bradycardia or to a heart rate of ≥60 bpm and evaluate concomitant medications known to cause bradycardia, as well as anti-hypertensive medications |
| • |
If attributable to a concomitant medication, resume ALECENSA at a reduced dose upon recovery to asymptomatic bradycardia or to a heart rate of ≥60 bpm, with frequent monitoring as clinically indicated |
| • |
Permanently discontinue ALECENSA in case of recurrence or in cases of life-threatening bradycardia if no contributing concomitant medication is identified |
|
| Severe Myalgia and Creatine Phosphokinase (CPK) Elevation |
| • |
Myalgia or musculoskeletal pain occurred in 26% of patients. The incidence of Grade 3 myalgia/musculoskeletal pain was 0.7%. Dose modifications for myalgia/musculoskeletal pain were required in 0.5% of patients |
| • |
Elevations of CPK occurred in 41% of 347 patients with CPK laboratory data. The incidence of Grade 3 elevations of CPK was 4.0%. Median time to Grade 3 CPK elevation was 14 days (interquartile range 13-28 days). Dose modifications for elevation of CPK occurred in 3.2% of patients |
| • |
Advise patients to report any unexplained muscle pain, tenderness, or weakness. Assess CPK levels every 2 weeks for the first month of treatment and as clinically indicated in patients reporting symptoms. Based on the severity of the CPK elevation, withhold, then resume or dose reduce ALECENSA |
|
| Embryo-Fetal Toxicity |
| • |
ALECENSA can cause fetal harm when administered to pregnant women. Administration of ALECENSA to pregnant rats and rabbits during the period of organogenesis resulted in embryo-fetal toxicity and abortion at maternally toxic doses with exposures approximately 2.7X those observed in humans with ALECENSA 600 mg twice daily. Advise pregnant women of the potential risk to a fetus |
| • |
Advise females of reproductive potential to use effective contraception during treatment with ALECENSA and for 1 week following the final dose |
| • |
Advise males with female partners of reproductive potential to use effective contraception during treatment with ALECENSA and for 3 months following the final dose |
|
|
|