|
|
| This white paper highlights the benefits of PKS Online™, an on-demand clinical pharmacology data repository from Pharsight® designed to connect global drug development teams. PKS Online offers a real-time portal to build and maintain the required database of pharmacokinetic and pharmacodynamic study data, analyses and regulatory reports for submission. Download… |
Strategic Benefits of an Online Clinical Data Repository
Complimentary White Paper |
|
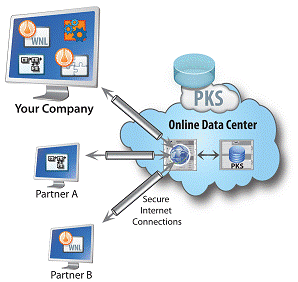
An emerging trend for many life sciences companies is the use of globally dispersed teams to run clinical studies, collect patient data and perform biostatistical and PK/PD analysis. One challenge resulting from this network of multiple partners and outsourcing relationships is the management and oversight of complex PK/PD studies that lack standardization. Regardless of company size, key questions faced by drug development organizations are:
- How to support optimized workflows for PK/PD analysis, modeling and reporting?
- How to efficiently provide high-quality, regulatory compliant storage for PK/PD data?
- How to speed data transfer and information exchange between and among global drug development partners?
This white paper introduces a solution that acts as the central hub to tie global team members together and offer a real time portal into data and reports that can be used to reduce timelines to regulatory submission, enhance data management for multi-study analysis, and support valuable clinical study data in a 'transaction-ready' state for due-diligence and out-licensing.
» Download Now
|
|
|
Presented by:
 |
|
|
|
|
|
If you no longer wish to receive emails from Pharsight, Please reply HERE
Pharsight, A Certara Company
5625 Dillard Dr., Suite 205
Cary, NC 27518
Tel: 919-859-6868 | Fax: 919-859-6871
ACT is published by Advanstar Communications, 485 Rt 1 South, Iselin, NJ 08830.
This is an advertisement sent on behalf of this advertiser. |
|