Q1 Review from Process Development Forum
Top Stories, Topics and News in the First Quarter of 2013
|
|
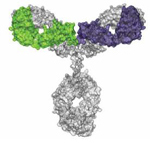
Development of a Purification Platform Process For a Unique Next-Gen Antibody
NovImmune, which pioneered κλ-body, a bispecific antibody format, has now designed a platform purification process for this novel antibody. Read more in a poster presented by the NovImmune team.
|
 |
 |
PDA's technical report for biotech cleaning validation
Quality by Design principles such as design space can also be applied to cleaning validation. As discussed in the PDA Technical Report No. 49: Points to Consider for Biotechnology Cleaning Validation, well–designed laboratory-scale studies can be performed using design of experiments, and the data analyzed to understand the cleaning process. With the knowledge of large-scale equipment, one can create an approach that results in a successful cleaning validation.Read more.
|
FDA Lists Guidance Documents Planned for 2013.
FDA has released a list of more than 50 guidance documents planned for 2013. The list reflects guidance documents currently under development as of the date of the posting, Jan. 31, 2013. With regards to biosimilars, the agency plans a guidance on Submission of Clinical Pharmacology Data as Evidence of Biosimilarity for Biologics and Protein Products, as well as one titled Formal Meetings Between the FDA and Biosimilar Biological Product Sponsors or Applicants. Continue reading.
|
 |
 |
Free Lunches Available Here.
GE Healthcare Life Sciences offers handbooks covering a wide range of techniques and applications including protein purification, antibody purification, chromatography, electrophoresis, cell culture and many other areas. Whether you’re a beginner or an expert, the handbooks are a great reference for practical tips and in-depth information about common methodologies used in the lab. Continue reading.
|
EU Sets Guidelines for Biosimilar Monoclonal Antibodies.
The European Union has reached an important stage in its efforts to make the region a biosimilars production center. The London based European Medicines Agency (EMA), which licenses EU pharmaceuticals, has finalized a guideline on biosimilar monoclonal antibodies (mAbs) and is drafting another one on the key issue of quality of biosimilars (1, 2).Read more.
|
 |
|
|
Questions for 2013
Like what you read? Want to see more of something in particular? Let us know what topics are on your mind and what you'd like to see more of in 2013. |
|
|
|
|

