| GAZYVA (obinutuzumab), in combination with chemotherapy followed by GAZYVA monotherapy in patients achieving at least a partial remission, is indicated for the treatment of adult patients with previously untreated stage II bulky, III or IV follicular lymphoma (FL). |
| This indication is based on the results of the Phase III, randomized GALLIUM trial, which studied GAZYVA and rituximab head-to-head in over 1,200 patients when each was combined with chemotherapy and followed by GAZYVA or rituximab monotherapy, respectively. |
| GALLIUM Trial: Efficacy |
| GALLIUM demonstrated that GAZYVA is the first and only approved therapy in previously untreated stage II bulky, III, or IV FL to deliver superior PFS vs rituximab when each was combined with chemotherapy* and followed by GAZYVA or rituximab monotherapy. The IRC assessment showed the GAZYVA regimen reduced risk of disease progression or death by 28% vs the rituximab regimen (HR=0.72; 95% CI, 0.56-0.93; P=0.0118; 38-month median observation time). Median PFS has not been reached in either arm of the trial. |
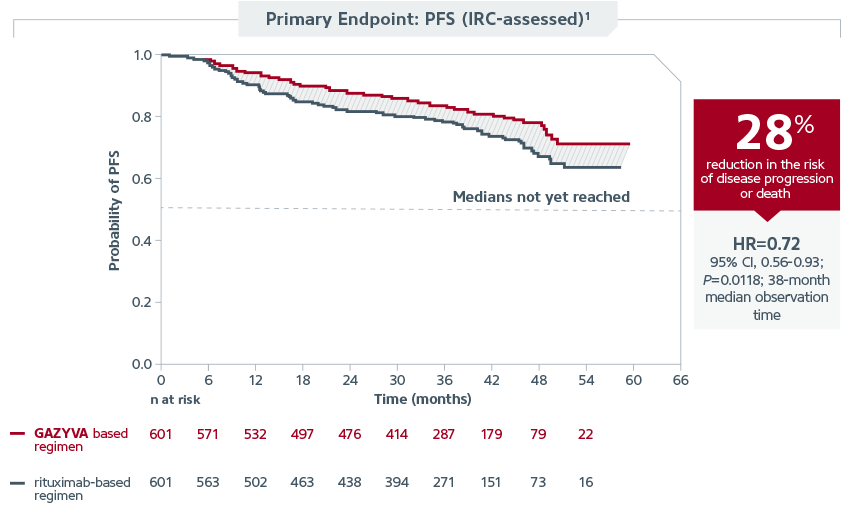 |
| GALLIUM Trial Design and Patient Population |
| Patients were randomized to receive GAZYVA (n=601) or rituximab (n=601) in combination with chemotherapy (CHOP, CVP, or bendamustine) for 6 or 8 cycles. Patients who achieved complete or partial response to the initial 6 or 8 cycles continued to receive GAZYVA or rituximab monotherapy, respectively, every 2 months until disease progression or for a maximum of 2 years. |
| The trial population (n=1,202) was inclusive of a broad range of previously untreated FL patient types, enrolling patients with disease Grades 1-3a, and stage II/III/IV, with 44% having bulky disease (≥7 cm) overall. Each investigator site chose to combine GAZYVA or rituximab with bendamustine (57%), CHOP (33%), or CVP (10%). All patients received the chosen chemotherapy at that site for the duration of the induction. |
| The treatment arms were generally balanced with respect to demographic factors and baseline disease characteristics.2 |
| GALLIUM Trial: Safety Information |
| Common adverse reactions (≥10% incidence and ≥2% greater in the GAZYVA arm), in patients with previously untreated NHL1 |
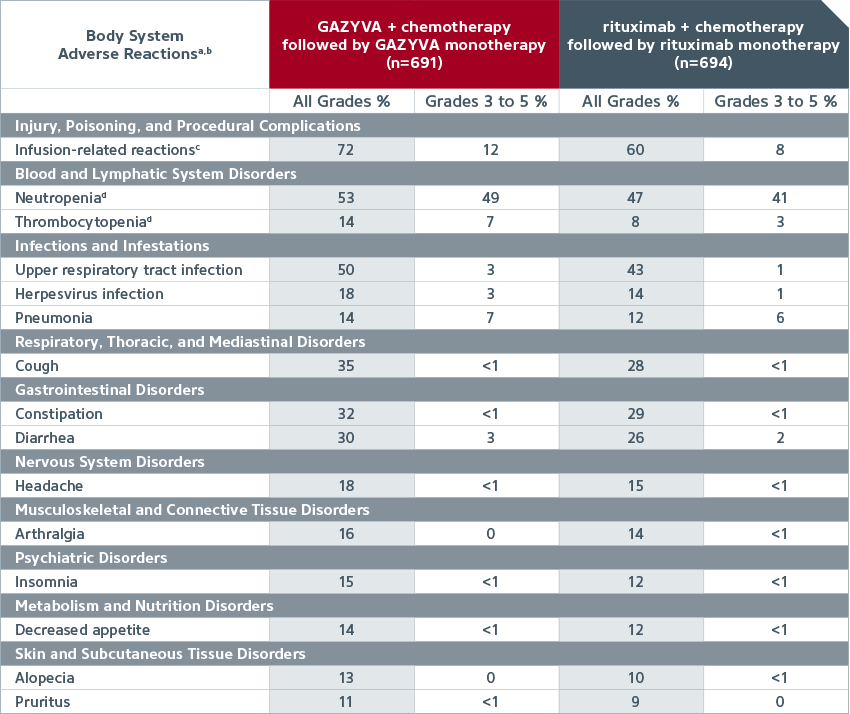 |
| A randomized, open-label multicenter trial (GALLIUM) evaluated the safety of GAZYVA as compared to rituximab product in 1,385 patients with previously untreated follicular lymphoma (86%) or marginal zone lymphoma (14%). |
| Common new or worsening laboratory abnormalities (≥10% incidence and ≥2% greater in the GAZYVA arm) in patients with previously untreated NHL1,a |
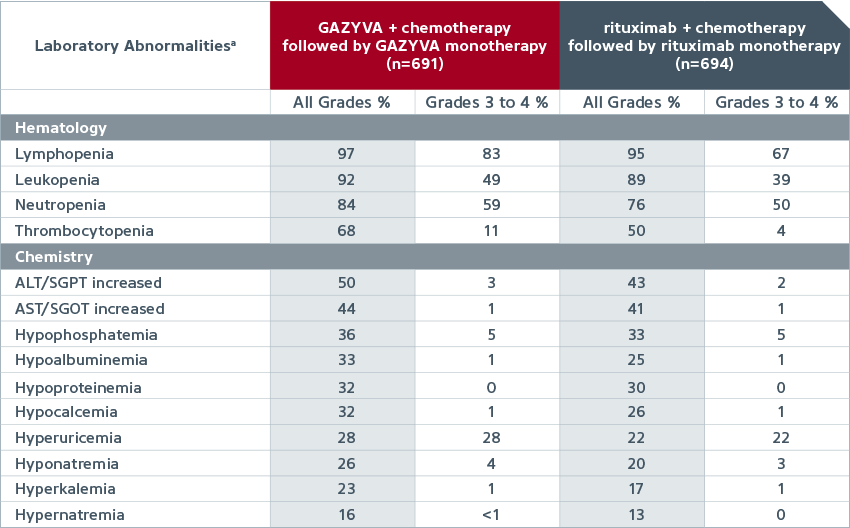 |
| GAZYVA monotherapy investigator-reported adverse reactions1 |
| • |
The common adverse reactions (incidence ≥10%) observed at least 2% more with GAZYVA were upper respiratory tract infection (40%), cough (23%), musculoskeletal pain (20%), neutropenia (19%) and herpes virus infection (13%) |
|
| GAZYVA monotherapy hematological laboratory abnormalities1 |
| • |
New-onset Grade 3 or 4 neutropenia was reported in 21% of patients in the GAZYVA arm (Grade 4, 10%) and 17% of patients in the rituximab product arm (Grade 4, 9%) |
|
| Adverse events leading to treatment withdrawal1 |
| • |
18% in the GAZYVA arm vs 15% in the rituximab arm |
|
| Select Trial Safety Information |
| • |
Infusion Reactions: Overall 72% of patients in the GAZYVA treated arm experienced an infusion reaction (all grades). The incidence of Grade 3 to 4 infusion reactions for these patients was 12%. In Cycle 1, the incidence of infusion reactions (all grades) was 62% in the GAZYVA treated arm with Grade 3 to 4 infusion reactions reported in 10%. The incidence of infusion reactions (all grades) was highest on Day 1 (60%) and decreased on Days 8 and 15 (9% and 6% respectively). During Cycle 2, the incidence of infusion reactions (all grades) in the GAZYVA treated arm was 13% and decreased with subsequent cycles. During GAZYVA monotherapy treatment, infusion reactions (all grades) were observed in 9% of patients. Overall, 1% of patients experienced an infusion reaction leading to discontinuation of GAZYVA |
| • |
Neutropenia: The incidence of neutropenia was higher in the GAZYVA treated arm (53%) compared to the rituximab product treated arm (47%). Cases of prolonged neutropenia (1%) and late onset neutropenia (4%) were also reported in the GAZYVA treated arm. The incidence of neutropenia was higher during treatment with GAZYVA in combination with chemotherapy (45%) compared to the GAZYVA monotherapy treatment phase (20%) |
| • |
Infection: The incidence of infection was 82% in the GAZYVA treated arm and 73% in the rituximab product treated arm, with Grade 3 to 4 events reported in 21% and 17%, respectively. In the GAZYVA arm, fatal infections occurred in 2% of patients compared to <1% in the rituximab product arm. The incidence of Grade 3 to 4 infections in the GAZYVA and rituximab product treated arms was lower in patients receiving GCSF prophylaxis (14%; 16%) compared with patients not receiving GCSF prophylaxis (24%; 18%). The incidence of fatal infections in patients receiving GCSF prophylaxis in the GAZYVA and rituximab product treated arms was 2% and 0%, respectively, and was 2% and <1% in patients not receiving GCSF prophylaxis |
| • |
Thrombocytopenia: Thrombocytopenia was reported as an adverse reaction in 14% of the GAZYVA treated arm and 8% of the rituximab product treated arm, with the incidence of Grade 3 to 4 events being 7% and 3% respectively. The difference in incidences between the treatment arms is driven by events occurring during the first cycle. The incidence of thrombocytopenia (all grades) in the first cycle were 9% in the GAZYVA and 3% in the rituximab product treated arms, with Grade 3 to 4 rates being 5% and 1%, respectively. Both treatment arms had a 12% overall incidence of hemorrhagic events and a <1% incidence of fatal hemorrhagic events |
| • |
Tumor Lysis Syndrome (TLS): The incidence of Grade 3 or 4 TLS was 0.9% in the GAZYVA treated arm |
| • |
Musculoskeletal Disorders: Musculoskeletal disorders were reported in 54% of patients in the GAZYVA treated arm and 49% of patients in the rituximab product treated arm |
| • |
Gastrointestinal Perforation: Cases of gastrointestinal perforation have been reported in patients receiving GAZYVA, mainly in NHL |
| • |
Worsening of Pre-existing Cardiac Conditions: Fatal cardiac events have been reported in patients treated with GAZYVA |
|
| For more information on the efficacy and safety of GAZYVA in previously untreated FL, visit our website. |
|
|
|
|
| Important Safety Information |
| BOXED WARNINGS: HEPATITIS B VIRUS REACTIVATION AND PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY |
| • |
Hepatitis B Virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients receiving CD20-directed cytolytic antibodies, including GAZYVA. Screen all patients for HBV infection before treatment initiation. Monitor HBV positive patients during and after treatment with GAZYVA. Discontinue GAZYVA and concomitant medications in the event of HBV reactivation |
| • |
Progressive Multifocal Leukoencephalopathy (PML) including fatal PML, can occur in patients receiving GAZYVA |
|
| Contraindications |
| • |
GAZYVA is contraindicated in patients with known hypersensitivity reactions (e.g. anaphylaxis) to obinutuzumab or to any of the excipients, or serum sickness with prior obinutuzumab use |
|
| Warnings and Precautions |
| Hepatitis B Virus (HBV) Reactivation |
| • |
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with anti-CD20 antibodies including GAZYVA. HBV reactivation has been reported in patients who are hepatitis B surface antigen (HBsAg) positive and in patients who are HBsAg negative but are hepatitis B core antibody (anti-HBc) positive. Reactivation has also occurred in patients who appear to have resolved hepatitis B infection (ie, HBsAg negative, anti-HBc positive, and hepatitis B surface antibody [anti-HBs] positive) |
| • |
HBV reactivation is defined as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level, or detection of HBsAg in a person who was previously HBsAg negative and anti-HBc positive. Reactivation of HBV replication is often followed by hepatitis, ie, increase in transaminase levels and, in severe cases, increase in bilirubin levels, liver failure, and death |
| • |
Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with GAZYVA. For patients who show evidence of hepatitis B infection (HBsAg positive [regardless of antibody status] or HBsAg negative but anti-HBc positive), consult physicians with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy |
| • |
Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months following treatment with GAZYVA |
| • |
In patients who develop reactivation of HBV while receiving GAZYVA, immediately discontinue GAZYVA and any concomitant chemotherapy and institute appropriate treatment. Resumption of GAZYVA in patients whose HBV reactivation resolves should be discussed with physicians with expertise in managing hepatitis B. Insufficient data exist regarding the safety of resuming GAZYVA in patients who develop HBV reactivation |
|
| Progressive Multifocal Leukoencephalopathy (PML) |
| • |
JC virus infection resulting in PML, which can be fatal, was observed in patients treated with GAZYVA. Consider the diagnosis of PML in any patient presenting with new onset or changes to preexisting neurologic manifestations. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture. Discontinue GAZYVA therapy and consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML |
|
| Infusion Reactions |
| • |
GAZYVA can cause severe and life-threatening infusion reactions. Sixty percent of patients with previously untreated NHL experienced a reaction on Day 1 of GAZYVA infusion. Infusion reactions can also occur with subsequent infusions. Symptoms may include hypotension, tachycardia, dyspnea, and respiratory symptoms (e.g., bronchospasm, larynx and throat irritation, wheezing, and laryngeal edema). Most frequently reported symptoms include nausea, fatigue, chest discomfort, dyspnea, dizziness, vomiting, diarrhea, rash, hypertension, hypotension, flushing, headache, pyrexia, and chills |
| • |
Premedicate patients with acetaminophen, an antihistamine, and a glucocorticoid. Institute medical management for infusion reactions as needed. Closely monitor patients during the entire infusion. Infusion reactions within 24 hours of receiving GAZYVA have occurred |
| • |
For patients with any Grade 4 infusion reactions, including but not limited to anaphylaxis, acute life-threatening respiratory symptoms, or other life-threatening infusion reaction: Stop the GAZYVA infusion. Permanently discontinue GAZYVA therapy |
| • |
For patients with Grade 1, 2, or 3 infusion reactions: Interrupt GAZYVA for Grade 3 reactions until resolution of symptoms. Interrupt or reduce the rate of the infusion for Grade 1 or 2 reactions and manage symptoms |
| • |
For patients with preexisting cardiac or pulmonary conditions, monitor more frequently throughout the infusion and the post-infusion period since they may be at greater risk of experiencing more severe reactions. Hypotension may occur as part of the GAZYVA infusion reaction. Consider withholding antihypertensive treatments for 12 hours prior to and during each GAZYVA infusion, and for the first hour after administration until blood pressure is stable. For patients at increased risk of hypertensive crisis, consider the benefits versus the risks of withholding their antihypertensive medication |
|
| Hypersensitivity Reactions Including Serum Sickness |
| • |
Hypersensitivity reactions have been reported in patients treated with GAZYVA. Signs of immediate-onset hypersensitivity included dyspnea, bronchospasm, hypotension, urticaria and tachycardia. Late-onset hypersensitivity diagnosed as serum sickness has also been reported with symptoms that include chest pain, diffuse arthralgia and fever. Hypersensitivity reactions may be difficult to clinically distinguish from infusion related reactions. However, hypersensitivity very rarely occurs with the first infusion and, when observed, often occur after previous exposure. If a hypersensitivity reaction is suspected during or after an infusion, the infusion must be stopped and treatment permanently discontinued. Patients with known hypersensitivity reactions to GAZYVA, including serum sickness, must not be retreated |
|
| Tumor Lysis Syndrome (TLS) |
| • |
Tumor lysis syndrome, including fatal cases, has been reported in patients receiving GAZYVA. Patients with high tumor burden, high circulating lymphocyte count (>25 x 109/L) or renal impairment are at greater risk for TLS and should receive appropriate tumor lysis prophylaxis with antihyperuricemics (eg, allopurinol or rasburicase) and hydration prior to the infusion of GAZYVA. During the initial days of GAZYVA treatment, monitor the laboratory parameters of patients considered at risk for TLS. For treatment of TLS, correct electrolyte abnormalities, monitor renal function and fluid balance, and administer supportive care, including dialysis as indicated |
|
| Infections |
| • |
Fatal and serious bacterial, fungal, and new or reactivated viral infections can occur during and following GAZYVA therapy. When GAZYVA is administered with chemotherapy followed by GAZYVA monotherapy, Grade 3 to 5 infections have been reported in up to 8% of patients during combination therapy, up to 13% of patients during monotherapy, and up to 8% of patients after treatment. Do not administer GAZYVA to patients with an active infection. Patients with a history of recurring or chronic infections may be at increased risk of infection |
| • |
In GALLIUM, more Grade 3 to 5 infections were reported in the recipients of GAZYVA and bendamustine (117/410 patients, 29%), as compared to GAZYVA plus CHOP or CVP (43/281 patients, 15%). More fatal infections were reported in patients treated with GAZYVA and bendamustine (3%), as compared to GAZYVA plus CHOP or CVP (<1%), including during the monotherapy phase and after completion of treatment |
|
| Neutropenia |
| • |
Severe and life-threatening neutropenia, including febrile neutropenia, has been reported during treatment with GAZYVA. Monitor patients with Grade 3 to 4 neutropenia frequently with regular laboratory tests until resolution. Anticipate, evaluate, and treat any symptoms or signs of developing infection. Consider administration of granulocyte colony-stimulating factors (GCSF) in patients with Grade 3 or 4 neutropenia |
| • |
Neutropenia can also be of late onset (occurring more than 28 days after completion of treatment) and/or prolonged (lasting longer than 28 days) |
| • |
Consider dose delays in the case of Grade 3 or 4 neutropenia. Patients with severe and long lasting (>1 week) neutropenia are strongly recommended to receive antimicrobial prophylaxis until resolution of neutropenia to Grade 1 or 2. Consider antiviral and antifungal prophylaxis |
|
| Thrombocytopenia |
| • |
Severe and life threatening thrombocytopenia has been reported during treatment with GAZYVA in combination with chemotherapy. Fatal hemorrhagic events have been reported in patients with NHL treated with GAZYVA in combination with chemotherapy, including during Cycle 1. Monitor all patients frequently for thrombocytopenia and hemorrhagic events, especially during the first cycle. In patients with Grade 3 or 4 thrombocytopenia, monitor platelet counts more frequently until resolution and consider subsequent dose delays of GAZYVA and chemotherapy or dose reductions of chemotherapy. Transfusion of blood products (i.e., platelet transfusion) may be necessary. Consider withholding concomitant medications which may increase bleeding risk (platelet inhibitors or anticoagulants), especially during the first cycle |
|
| Immunization |
| • |
The safety and efficacy of immunization with live or attenuated viral vaccines during or following GAZYVA therapy have not been studied. Immunization with live virus vaccines is not recommended during treatment and until B-cell recovery |
|
| Pregnancy |
| • |
There are no data with GAZYVA use in pregnant women to inform a drug-associated risk. GAZYVA is likely to cause fetal B-cell depletion. GAZYVA should be used during pregnancy and/or breastfeeding only if the potential benefit justifies the potential risk to the fetus and/or infant. Mothers who have been exposed to GAZYVA during pregnancy should discuss the safety and timing of live virus vaccinations for their infants with their child’s healthcare providers |
|
| Geriatric Use |
| • |
Of the 691 patients in GALLIUM treated with GAZYVA plus chemotherapy as first-line therapy, 33% were 65 and over, while 7% were 75 and over. Of patients 65 and over, 63% experienced serious adverse reactions and 26% experienced adverse reactions leading to treatment withdrawal, while in patients under 65, 43% experienced serious adverse reactions and 13% had an adverse reaction leading to treatment withdrawal. No clinically meaningful differences in efficacy were observed between these patients and younger patients |
|
| Additional Important Safety Information |
| • |
A randomized, open-label multicenter trial (GALLIUM) evaluated the safety of GAZYVA as compared to rituximab product in 1,385 patients with previously untreated follicular lymphoma (86%) or marginal zone lymphoma (14%) |
| • |
Serious adverse reactions occurred in 50% of patients on the GAZYVA arm and 43% of patients on the rituximab product arm. Fatal adverse reactions were reported during treatment in 3% in the GAZYVA arm and 2% in the rituximab product arm, most often from infections in the GAZYVA arm. During treatment and follow-up combined, fatal adverse reactions were reported in 5% of the GAZYVA arm and 4% of the rituximab product arm, with infections and second malignancies being leading causes. In the GAZYVA arm, fatal infections occurred in 2% of patients compared to <1% in the rituximab product arm |
| • |
Neutropenia, infusion related reactions, febrile neutropenia and thrombocytopenia were the most common Grade 3 to 5 adverse reactions (incidence ≥5%) observed more frequently in the GAZYVA arm |
|
|
|
|
|
| • |
Throughout treatment and follow-up, the most common adverse reactions (incidence ≥20%) observed at least 2% more in the GAZYVA arm were infusion related reactions, neutropenia, upper respiratory tract infection, cough, constipation and diarrhea |
| • |
During the monotherapy period, the common adverse reactions (incidence ≥10%) observed at least 2% more with GAZYVA were upper respiratory infection (40%), cough (23%), musculoskeletal pain (20%), neutropenia (19%) and herpesvirus infection (13%) |
|
| You are encouraged to report side effects to Genentech and the FDA. You may contact Genentech by calling 1-888-835-2555. You may contact the FDA by visiting www.fda.gov/medwatch, or calling 1-800-FDA-1088. |
| Please see the full Prescribing Information for additional Important Safety Information, including BOXED WARNINGS. |
|
|
|
|
|