Give adults with type 2 diabetes improved A1C control
To ensure receipt of future emails, please add NovoNordisk@email.novonordiskmail.com to your address book.
To ensure receipt of future emails, please add NovoNordisk@email.novonordiskmail.com to your address book.

IN A 52-WEEK STUDY AS MONOTHERAPY
Victoza®—improved A1C control with daily management of FPG and PPG.1
|
 |
When it comes to managing type 2 diabetes in adults every day, consider turning to the #1 prescribed GLP-1 receptor agonist therapy. Victoza® is a once-daily injection that may provide your patients significant A1C reductions as well as daily management of fasting plasma glucose (FPG) and postprandial glucose (PPG).
Victoza® is approved for use after 1 or more oral antidiabetic drugs and in combination with basal insulin. Victoza® is not recommended as first-line therapy in patients inadequately controlled on diet and exercise.
Ordering Information
Victoza® (liraglutide [rDNA origin] injection) |
2-Pen Package |
3-Pen Package |
| NDC Number | 0169-4060-12 | 0169-4060-13 |
| AmerisourceBergen Item Number | 10000349 | 10000356 |
| Cardinal Item Number | 4279311 | 4279328 |
| McKesson Item Number | 1891332 | 1918473 |
| Selected Important Safety Information | ||||||||
|
||||||||
| Please click here to see additional Important Safety Information below. | ||||||||
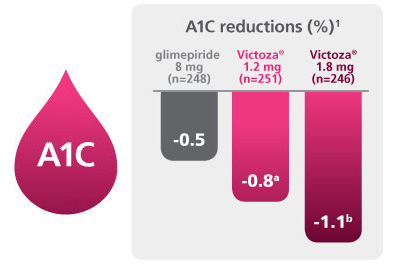
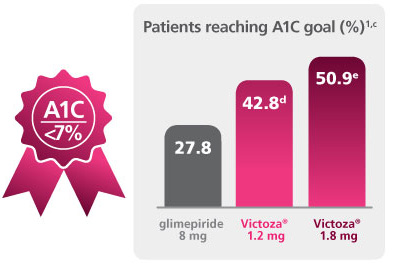
Significant A1C reductions
|
| FPG reductions | |
| FPG reductions were seen at 2 weeks and sustained for 52 weeks.1 | |
| • | -25.6 mg/dL with Victoza® 1.8 mgf |
| • | -15.1 mg/dL with Victoza® 1.2 mgg |
| • | -5.2 mg/dL with glimepiride 8 mg |
| Lower PPG | |
| Victoza® lowered PPG throughout the day.1 | |
| • | -37.4 mg/dL with Victoza® 1.8 mgh |
| • | -30.8 mg/dL with Victoza® 1.2 mgi |
| • | -24.5 mg/dL with glimepiride 8 mg |
PPG was measured as a secondary endpoint in clinical studies. PPG data were obtained via self-monitoring of blood glucose.
Victoza® lowered fasting, premeal, and postprandial glucose throughout the day.
A 52-week, double-blind, double-dummy, active-controlled, study. Intention-to-treat patients (N=745) received once-daily Victoza® 1.2 mg (n=251), Victoza® 1.8 mg (n=246), or glimepiride 8 mg (n=248). The primary outcome was change in A1C after 52 weeks.1
a P<0.05 vs comparator.
b P<0.0001 vs comparator. c ADA A1C goal is <7%. d P=0.0007 vs comparator. e P<0.0001 vs comparator. f P=0.0001 vs comparator. g P=0.027 vs comparator. h P=0.0038 vs comparator. i P=0.1616 vs comparator. Victoza® is not recommended as first-line therapy in patients inadequately controlled on diet and exercise.
|
|
| Adverse Events in the 52-Week Study | |
| • | Adverse reactions with Victoza® (n=497) vs glimepiride (n=248): nausea (28.4% vs 8.5%), diarrhea (17.1% vs 8.9%), vomiting (10.9% vs 3.6%), constipation (9.9% vs 4.8%), and headache (9.1% vs 9.3%) |
| • | No major hypoglycemic events occurred. There was a low rate of minor hypoglycemia compared with glimepiride (Victoza® 1.2 mg: 12.0%; Victoza® 1.8 mg: 8.0%; glimepiride 8 mg: 24.0%)1 |
|
VictozaCare™ helps patients start on Victoza® and stay on track with tools and support. Patients who enrolled in VictozaCare™ were more adherent than those not enrolled.2,j
Encourage your patients to call 844-295-7206 or go to connect.victozacare.com to enroll in VictozaCare™
j Crossix ScoreBoard™ Report, May 2013. Adherence measured by number of actual Victoza® prescriptions filled for existing Victoza® patients enrolled in VictozaCare™, versus a match-pair control group not enrolled in VictozaCare™, through up to the first 17 months of enrollment.
|
|
| Indications and Usage | ||||||||
| • | Victoza® is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. | |||||||
| Important Limitations of Use: | ||||||||
| • | Victoza® is not recommended as first-line therapy for patients who have inadequate glycemic control on diet and exercise because of the uncertain relevance of the rodent C-cell tumor findings to humans. Prescribe Victoza® only to patients for whom the potential benefits are considered to outweigh the potential risk. | |||||||
| • | Based on spontaneous postmarketing reports, acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with Victoza®. Victoza® has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at increased risk for pancreatitis while using Victoza®. Other antidiabetic therapies should be considered in patients with a history of pancreatitis. | |||||||
| • | Victoza® is not a substitute for insulin. Victoza® should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings. | |||||||
| • | The concurrent use of Victoza® and prandial insulin has not been studied. | |||||||
| Important Safety Information | ||||||||
|
||||||||
| Contraindications | ||||||||
| • | Victoza® is contraindicated in patients with a prior serious hypersensitivity reaction to Victoza® or to any of the product components. | |||||||
| Warnings and Precautions | ||||||||
| • | Pancreatitis: Based on spontaneous postmarketing reports, acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with Victoza®. After initiation of Victoza®, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, Victoza® should promptly be discontinued and appropriate management should be initiated. If pancreatitis is confirmed, Victoza® should not be restarted. Consider antidiabetic therapies other than Victoza® in patients with a history of pancreatitis. | |||||||
| • | Never Share a Victoza® Pen Between Patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens. | |||||||
| • | Use with Medications Known to Cause Hypoglycemia: When Victoza® is used with an insulin secretagogue (e.g. a sulfonylurea) or insulin, serious hypoglycemia can occur. Consider lowering the dose of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. | |||||||
| • | Renal Impairment: Renal impairment has been reported postmarketing, usually in association with nausea, vomiting, diarrhea, or dehydration, which may sometimes require hemodialysis. Use caution when initiating or escalating doses of Victoza® in patients with renal impairment. | |||||||
| • | Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g. anaphylaxis and angioedema) have been reported postmarketing. If symptoms of hypersensitivity reactions occur, patients must stop taking Victoza® and seek medical advice promptly. | |||||||
| • | Macrovascular Outcomes: There have been no studies establishing conclusive evidence of macrovascular risk reduction with Victoza® or any other antidiabetic drug. | |||||||
| Adverse Reactions: | ||||||||
| • | The most common adverse reactions, reported in ≥5% of patients treated with Victoza® and more commonly than in patients treated with placebo, are headache, nausea, diarrhea, dyspepsia, constipation, and anti-liraglutide antibody formation. Immunogenicity-related events, including urticaria, were more common among Victoza®-treated patients (0.8%) than among comparator-treated patients (0.4%) in clinical trials. | |||||||
| Use in Specific Populations: | ||||||||
| • | Victoza® has not been studied in patients with type 2 diabetes below 18 years of age and is not recommended for use in pediatric patients. | |||||||
| • | There is limited data in patients with renal or hepatic impairment. | |||||||
| Please click here for Prescribing Information. | ||||||||
For more information about Victoza®, please visit VictozaPro.com
To unsubscribe from future communications from Novo Nordisk Inc., please click here. Additionally, you can unsubscribe from future Novo Nordisk communications by calling 1-877-744-2579 or sending a brief note with your name and address to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536.
References: 1. Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. 2. Crossix Solutions Inc. Crossix ScoreBoard™, May 2013.
Victoza® is a registered trademark and VictozaCare™ is a trademark of Novo Nordisk A/S.
© 2015 Novo Nordisk All rights reserved. 0815-00028239-1 October 2015Novo Nordisk is a registered trademark of Novo Nordisk A/S. |
||