|
In a recent survey, ClearTrial polled 187 professionals from 115 small, mid-sized, and Top 20 biopharmaceutical and medical device companies. Each person–representing clinical operations, outsourcing, finance, and project management—was asked specific questions regarding their forecasting and budgeting practices and metrics.
I am pleased to present the consolidated results of this survey in the report, “Clinical Study Budgeting Practices and Metrics.”
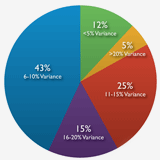
Among other findings, almost half the survey respondents reported that their typical variance—from forecast to actual costs—for clinical studies was at least 11%, and was often greater. An astonishing one in five stated their cost variance was 16% or more.
Download “Clinical Study Budgeting Practices and Metrics” today for the complete report, and to discover how you compare with your peers in this important area.
Don't hesitate to contact me with any questions about the survey results.
Best regards,
Catherine Ginzer
cginzer@cleartrial.com
+1 312.460.3018
|
 |
|
What is your typical budget variance from forecast to actual at the study level?
|
|