|

|
| TREAT HER EARLY |
| Patients achieving pCR: Data from 2 clinical trials |
| Indication |
| PERJETA® (pertuzumab) is a HER2/neu receptor antagonist indicated for use in combination with Herceptin®
(trastuzumab) and docetaxel as neoadjuvant treatment of patients with
HER2‑positive, locally advanced, inflammatory, or early stage breast
cancer (either greater than 2 cm in diameter or node positive) as part
of a complete treatment regimen for early breast cancer. This indication
is based on demonstration of an improvement in pathological complete
response rate. No data are available demonstrating improvement in
event-free survival or overall survival. |
| Limitations of Use: |
| • |
The safety of PERJETA as part of a doxorubicin‑containing regimen has not been established |
| • |
The safety of PERJETA administered for greater than 6 cycles for early breast cancer has not been established |
|
|
| Consider
a PERJETA and Herceptin combination-based neoadjuvant regimen for all
eligible patients with HER2+ early-stage breast cancer (positive nodal
status or >2 cm tumor)1,2 |
| Patient Eligibility by Breast Cancer Stage1,2 |

|
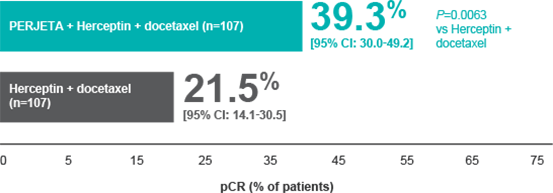
| NeoSphere
Efficacy: Nearly doubled pathological complete response (pCR)* rates
with PERJETA + Herceptin + docetaxel vs Herceptin + docetaxel1 |
| Percentage of patients who achieved pCR in breast and nodes (FDA-preferred endpoint) in the NeoSphere trial† |
|
|
| Indication |
| PERJETA® (pertuzumab) is a HER2/neu receptor antagonist indicated for use in combination with Herceptin®
(trastuzumab) and docetaxel as neoadjuvant treatment of patients with
HER2‑positive, locally advanced, inflammatory, or early stage breast
cancer (either greater than 2 cm in diameter or node positive) as part
of a complete treatment regimen for early breast cancer. This indication
is based on demonstration of an improvement in pathological complete
response rate. No data are available demonstrating improvement in
event-free survival or overall survival. |
| Limitations of Use: |
| • |
The safety of PERJETA as part of a doxorubicin-containing regimen has not been established |
| • |
The safety of PERJETA administered for greater than 6 cycles for early breast cancer has not been established |
|
|
|
|
|
|
| • |
PERJETA + Herceptin (n=107): 11.2% pCR (95% CI: 5.9-18.8; P=0.0223) vs Herceptin + docetaxel1 |
| • |
PERJETA + docetaxel (n=96): 17.7% pCR (95% CI: 10.7-26.8; P=0.0018) vs PERJETA + Herceptin + docetaxel1 |
|
| Percentage of patients in subgroups who achieved pCR in breast and nodes1 |
| • | In the hormone receptor–positive subgroup | | – | 22% (95% CI: 11.5-36.0) for PERJETA + Herceptin + docetaxel (n=50) | | – | 12% (95% CI: 4.5-24.3) for Herceptin + docetaxel (n=50) | | – | 2.0% (95% CI: 0.1-10.5) for PERJETA + Herceptin (n=51‡) | | – | 8.7% (95% CI: 2.4-20.8) for PERJETA + docetaxel (n=46) |
|
|
| • | In the hormone receptor–negative subgroup | | – | 54.4% (95% CI: 40.7-67.6) for PERJETA + Herceptin + docetaxel (n=57) | | – | 29.8% (95% CI: 18.4-43.4) for Herceptin + docetaxel (n=57) | | – | 20.0% (95% CI: 10.4-33.0) for PERJETA + Herceptin (n=55‡) | | – | 26.0% (95% CI: 14.6-40.3) for PERJETA + docetaxel (n=50) |
|
|
|
|
| Important Safety Information |
| Boxed WARNINGS: Left Ventricular Dysfunction and Embryo-Fetal Toxicity |
| • |
PERJETA
administration can result in subclinical and clinical cardiac failure
manifesting as decreased LVEF and CHF. Evaluate cardiac function prior
to and during treatment. Discontinue PERJETA treatment for a confirmed
clinically significant decrease in left ventricular function |
| • |
Exposure
to PERJETA can result in embryo-fetal death and birth defects. Advise
patients of these risks and the need for effective contraception |
|
| Please see additional select Important Safety Information throughout, and the accompanying full Prescribing Information, including Boxed WARNINGS. |
| NeoSphere cardiac safety profile1 |
| • |
In
the neoadjuvant treatment period, left ventricular dysfunction (LVD)
occurred in 2.8% of patients treated with PERJETA + Herceptin +
docetaxel, in 0.9% with Herceptin + docetaxel, in 0.0% with PERJETA +
Herceptin, and in 1.1% with PERJETA + docetaxel |
| – |
Symptomatic
LVD congestive heart failure (CHF) occurred in 0.9% of patients treated
with PERJETA + Herceptin and in 0.0% with PERJETA + Herceptin +
docetaxel, Herceptin + docetaxel, and PERJETA + docetaxel |
|
| • |
In the overall treatment
period, left ventricular ejection fraction (LVEF) decline >10% and a
drop to less than 50% occurred in 1.9% of patients treated with
Herceptin + docetaxel vs 8.4% of patients treated with PERJETA +
Herceptin + docetaxel. LVEF recovered to ≥50% in all patients |
|
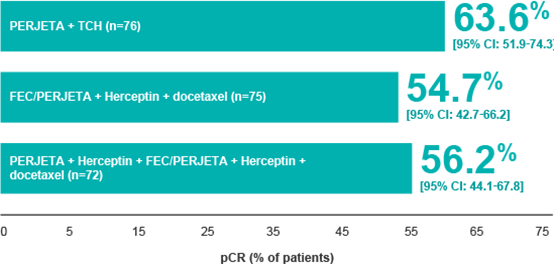
| TRYPHAENA Efficacy: pCR achieved in the majority of patients1 |
| Percentage of patients who achieved pCR in breast and nodes in the TRYPHAENA trial1§ |
|
| Percentage of patients in subgroups who achieved pCR in breast and nodes1 |
| • | In the hormone receptor–positive subgroup | | – | 47.5% (95% CI: 31.5-63.9) for PERJETA + TCH | | – | 45.7% (95% CI: 28.8-63.4) for FEC followed by PERJETA + Herceptin + docetaxel | | – | 41.0% (95% CI: 25.6-57.9) for PERJETA + Herceptin + FEC followed by PERJETA + Herceptin + docetaxel |
|
| | • | In the hormone receptor–negative subgroup | | – | 81.1% (95% CI: 64.8-92.0) for PERJETA + TCH | | – | 62.5% (95% CI: 45.8-77.3) for FEC followed by PERJETA + Herceptin + docetaxel | | – | 73.5% (95% CI: 55.6-87.1) for PERJETA + Herceptin + FEC followed by PERJETA + Herceptin + docetaxel |
|
|
| TRYPHAENA trial endpoints1,4 |
| • |
Primary
endpoints: cardiac safety and tolerability during neoadjuvant
treatment, including incidence of symptomatic left ventricular systolic
dysfunction (LVSD) and decline in LVEF ≥10% from baseline to <50% |
| • |
Secondary endpoints: pCR in breast
(ypT0/is) assessed at surgery and additional pCR endpoint (basis for FDA
approval), pCR in breast and nodes (ypT0/is ypN0) assessed at surgery ‖¶ |
|
| TRYPHAENA cardiac safety profile1 |
| • |
In
the preoperative treatment period, LVD occurred in 5.6% of patients
treated with PERJETA + Herceptin + FEC/PERJETA + Herceptin + docetaxel,
in 4.0% of patients treated with FEC/PERJETA + Herceptin + docetaxel,
and in 2.6% of patients treated with PERJETA + TCH |
| • |
Symptomatic LVD (CHF) occurred in
2.7% of patients treated with FEC/PERJETA + Herceptin + docetaxel, in
0.0% of patients treated with PERJETA + TCH, and in 0.0% of patients
treated with PERJETA + Herceptin + FEC/PERJETA + Herceptin + docetaxel |
|
|
|
| Safety profile of PERJETA was evaluated in 2 clinical trials |
| NeoSphere Trial: AR incidence (all grades)1 |
| Most common (>20%) adverse reactions (ARs) all grades |
|
|
|
|
| TRYPHAENA Trial: AR incidence1 |
| The most common ARs (NCI-CTCAE v3.0) in TRYPHAENA with1: |
| PERJETA + TCH (n=76):
All grades (>30%) were fatigue, alopecia, diarrhea, nausea,
vomiting, neutropenia, thrombocytopenia, and anemia; Grades 3-4 (>2%)
were neutropenia, febrile neutropenia, anemia, leukopenia, diarrhea,
thrombocytopenia, vomiting, fatigue, ALT increased, hypokalemia, and
hypersensitivity. FEC/PERJETA + Herceptin + docetaxel (n=75):
All grades (>30%) were fatigue, alopecia, diarrhea, nausea,
vomiting, and neutropenia; Grades 3-4 (>2%) were neutropenia,
leukopenia, febrile neutropenia, diarrhea, LVD, anemia, dyspnea, nausea,
and vomiting. PERJETA + Herceptin + FEC/PERJETA + Herceptin + docetaxel (n=72):
All grades (>30%) were fatigue, alopecia, diarrhea, nausea,
vomiting, and neutropenia; Grades 3-4 (>2%) were neutropenia,
leukopenia, febrile neutropenia, diarrhea, and hypersensitivity |
|
|
| Important Safety Information |
| Boxed WARNINGS: Left Ventricular Dysfunction and Embryo-Fetal Toxicity |
| • |
PERJETA
administration can result in subclinical and clinical cardiac failure
manifesting as decreased LVEF and CHF. Evaluate cardiac function prior
to and during treatment. Discontinue PERJETA treatment for a confirmed
clinically significant decrease in left ventricular function |
| • |
Exposure
to PERJETA can result in embryo-fetal death and birth defects. Advise
patients of these risks and the need for effective contraception |
| – |
Verify
the pregnancy status of females of reproductive potential prior to the
initiation of PERJETA. Advise pregnant women and females of reproductive
potential that exposure to PERJETA in combination with trastuzumab
during pregnancy or within 7 months
prior to conception can result in fetal harm, including embryo‑fetal
death or birth defects. Advise females of reproductive potential to use
effective contraception during treatment and for 7 months following the
last dose of PERJETA in combination with trastuzumab |
| – |
There
is a pregnancy exposure registry that monitors pregnancy outcomes in
women exposed to PERJETA during pregnancy. Encourage women who receive
PERJETA in combination with trastuzumab during pregnancy or within 7 months prior to conception, to enroll in the MotHER Pregnancy Registry by contacting 1‑800‑690‑6720 or visiting http://www.motherpregnancyregistry.com/ |
| – |
If PERJETA is administered during pregnancy, or if a patient becomes pregnant while receiving PERJETA or within 7 months
following the last dose of PERJETA in combination with trastuzumab,
health care providers and patients should immediately report PERJETA
exposure to Genentech at 1‑888‑835‑2555 |
|
|
| Additional Important Safety Information |
| PERJETA is contraindicated in patients with known hypersensitivity to pertuzumab or to any of its excipients. |
| Left Ventricular Dysfunction (LVD) |
| • |
In
Study 1, for patients with MBC, left ventricular dysfunction, which
includes symptomatic left ventricular systolic dysfunction (LVSD)
(congestive heart failure) and decreases in left ventricular ejection
fraction (LVEF), occurred in 4.4% of patients in the PERJETA-treated
group and in 8.3% of patients in the placebo-treated group |
| • |
In
Study 2, for patients receiving neoadjuvant treatment, the incidence of
LVSD was higher in PERJETA-treated groups than in the trastuzumab and
docetaxel group. An increased incidence of LVEF declines was observed in
patients treated with PERJETA in combination with trastuzumab and
docetaxel. In the overall treatment period, LVEF decline >10% and a
drop to less than 50% occurred in 1.9% of patients treated with
neoadjuvant trastuzumab and docetaxel as compared to 8.4% of patients
treated with neoadjuvant PERJETA in combination with trastuzumab and
docetaxel |
| • |
In
Study 3, for patients receiving neoadjuvant treatment, in the overall
treatment period, LVEF decline >10% and a drop to less than 50%
occurred in 6.9% of patients treated with PERJETA plus trastuzumab and
FEC followed by PERJETA plus trastuzumab and docetaxel, in 16.0% of
patients treated with PERJETA plus trastuzumab and docetaxel following
FEC, and in 10.5% of patients treated with PERJETA in combination with
TCH |
| • |
Assess LVEF prior to initiation of
PERJETA and at regular intervals (eg, every 3 months in the metastatic
setting and every 6 weeks in the neoadjuvant setting) during treatment
to ensure that LVEF is within your institution’s normal limits |
|
| • |
If LVEF is
<45%, or is 45% to 49% with a 10% or greater absolute decrease below
the pretreatment value, withhold PERJETA and trastuzumab and repeat LVEF
assessment within approximately 3 weeks. Discontinue PERJETA and
trastuzumab if LVEF has not improved or has declined further |
|
| Infusion-Associated Reactions |
| • |
PERJETA has been associated with infusion reactions |
| • |
In
Study 1, when all drugs were administered on the same day, the most
common infusion reactions in the PERJETA-treated group (≥1.0%) were
fatigue, dysgeusia, hypersensitivity, myalgia, and vomiting |
| • |
In
Study 2 and Study 3, PERJETA was administered on the same day as the
other study treatment drugs. Infusion reactions were consistent with
those observed in Study 1, with a majority of reactions being National
Cancer Institute - Common Terminology Criteria for Adverse Events
(NCI-CTCAE v3.0) Grades 1‑2 |
| • |
If a significant infusion reaction
occurs, slow or interrupt the infusion and administer appropriate
medical therapies. Monitor patients carefully until complete resolution
of signs and symptoms. Consider permanent discontinuation in patients
with severe infusion reactions |
|
| Hypersensitivity Reactions/Anaphylaxis |
| • |
In
Study 1, the overall frequency of hypersensitivity/anaphylaxis
reactions was 10.8% in the PERJETA‑treated group and 9.1% in the
placebo-treated group. The incidence of Grades 3‑4 reactions was 2.0%
and 2.5%, respectively, according to NCI-CTCAE (version 3) |
| • |
In Study 2 and Study 3, hypersensitivity/anaphylaxis events were consistent with those observed in Study 1 |
| • |
Patients should be observed closely
for hypersensitivity reactions. Severe hypersensitivity, including
anaphylaxis, has been observed in clinical trials of PERJETA.
Medications to treat such reactions, as well as emergency equipment,
should be available for immediate use |
|
| HER2 Testing |
| • |
Detection of
HER2 protein overexpression is necessary for selection of patients
appropriate for PERJETA therapy because these are the only patients
studied and for whom benefit has been shown |
|
| Most Common Adverse Reactions |
| Neoadjuvant Treatment of Breast Cancer |
| • |
The
most common adverse reactions (>30%) with PERJETA in combination
with trastuzumab and docetaxel were alopecia, diarrhea, nausea, and
neutropenia. The most common NCI-CTCAE v3.0 Grades 3‑4 adverse reactions
(>2%) were neutropenia, febrile neutropenia, leukopenia, and
diarrhea |
| • |
The
most common adverse reactions (>30%) with PERJETA in combination with
trastuzumab and docetaxel when given for 3 cycles following 3 cycles of
FEC were fatigue, alopecia, diarrhea, nausea, vomiting, and
neutropenia. The most common NCI-CTCAE (version 3) Grades 3‑4 adverse
reactions (>2%) were neutropenia, leukopenia, febrile neutropenia,
diarrhea, left ventricular dysfunction, anemia, dyspnea, nausea, and
vomiting |
| • |
The most common adverse reactions
(>30%) with PERJETA in combination with docetaxel, carboplatin, and
trastuzumab (TCH) for 6 cycles were fatigue, alopecia, diarrhea, nausea,
vomiting, neutropenia, thrombocytopenia, and anemia. The most common
NCI-CTCAE (version 3) Grades 3‑4 adverse reactions (>2%) were
neutropenia, febrile neutropenia, anemia, leukopenia, diarrhea,
thrombocytopenia, vomiting, fatigue, ALT increased, hypokalemia, and
hypersensitivity |
|
| You may report side effects to the FDA at 1‑800‑FDA‑1088 or www.fda.gov/medwatch. You may also report side effects to Genentech at 1‑888‑835‑2555. |
| Please see additional select Important Safety Information throughout, and the accompanying full Prescribing Information, including Boxed WARNINGS. |
|
|
|
|
|