|
| |
|
|
 |
|
| |
|
|
| |
 |
|
| |
| Very soon, USP <233> will require analysis of elemental contaminants in pharmaceutical products. Determining contaminant concentration can be complicated. Many modern drugs have complex compositions like enteric coatings of synthetic polymers or biopolymers resistant to acidic breakdown; standard digestion techniques struggle with these applications. Complying with USP <233> requires a sample preparation method that ensures complete sample digestion – regardless of the matrix. |
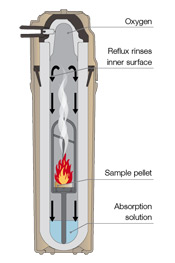 |
| Multiwave PRO from Anton Paar offers a unique microwave-induced oxygen combustion procedure, combining the advantages of ashing combustion with closed-vessel acid digestion, in one single preparation step. Used at NIST, this proven sample preparation method minimizes analyte losses and matrix effects. |
| Simplifying implementation, the 4Q qualification and validation package from Anton Paar fulfills GLP, GMP, GAMP 5, and USP <1058> guidelines and regulations – and is typically completed in less than 2 days. |
| |
| With sample digestion expertise and turnkey integration, Anton Paar is ready to help pharmaceutical manufacturers and analytical laboratories prepare for chapter USP <233> requirements. |
| Contact Anton Paar today to learn how! |
| |
|
| Anton Paar USA |
|
| 10215 Timber Ridge Drive |
|
| Ashland VA 23005 USA |
|
| |
|
| Tel.: (804) 550 1051 |
|
| Toll Free: (800) 722 7556 |
|
| Fax: (804) 550 1057 |
|
| |
|
| info.us@anton-paar.com |
|
| http://www.anton-paar.com |
© Anton Paar 2012 |
| |
| You are subscribed for the Anton Paar Newsletter. If you would like to unsubscribe to the Anton Paar Newsletter, click here: unsubscribe >> |
|
|
|
|
|
|