|
|
| |
|
| |
| |
|
|
| |
|
|
| |
Guest Speaker: Vanessa Figueroa |
|
DURATION: 1 Hour |
|
| |
|
|
| |
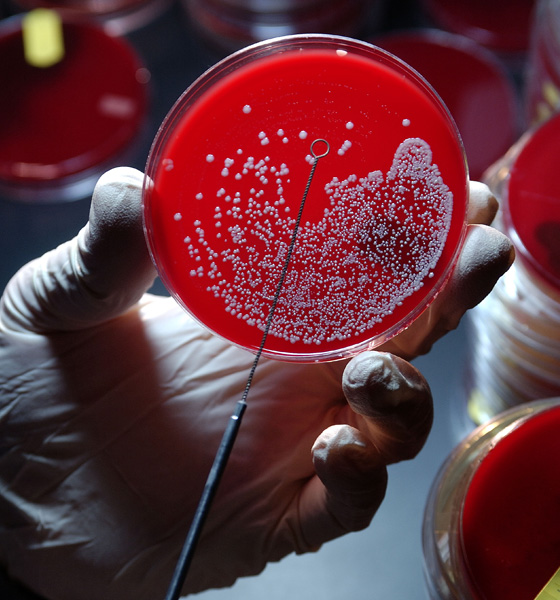
For most QA and QC labs, building a strong Environmental Monitoring program is a priority. If you want to learn how to design one that helps mitigate risk, watch the replay of our recent webinar, Best Practices for Environmental Monitoring and Risk Management. Our guest presenter, Vanessa Figueroa, provides an overview of key points to consider when designing an EM program that will help minimize risk and improve understanding of potential sources of contamination in your facility.
Don’t miss out on this critical information for setting up your EM program in a way that will help you reduce risk in the short and long term. |
|
| |
| |
|
|
|
|
| |
|
|
| |
| |
|
|
| |
|
|
| |
 |
|
Vanessa Figueroa, MA
Executive Director, Microbiology
Quality Executive Partners |
|
|
| |
|
|
| |
Vanessa has more than 12 years of combined experience in the pharmaceutical, biotechnology, and medical device industries. She has expertise in sterility assurance, environmental and utilities monitoring programs, and quality control laboratory management. Vanessa's consulting experiences have spanned both domestic and international large pharmaceutical companies, applying both US and global regulatory standards of quality. |
|
| |
|
|
| |
VIEW WEBINAR REPLAY → |
|
| |
|
|
|
|
| |
|
|
| |
|
|
| |
©2018 Charles River Laboratories International, Inc.
Charles River Laboratories | 1.877.274.8371 | 251 Ballardvale St. | Wilmington, MA | 01887
|
| |
|
|
|