|
|
|
|
|
The following promotional message is brought to you by Amgen, which is based on an analysis done by Amgen.
Persistence with Prolia® (denosumab) in postmenopausal osteoporosis patients was higher versus both IV and oral bisphosphonates at 3 years1
|
|
|
|
INDICATION
Prolia® is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy. In postmenopausal women with osteoporosis, Prolia® reduces the incidence of vertebral, nonvertebral, and hip fractures.
|
|
|
|
Dear [Insert Customer Name],
|
|

1 in 2 women over age 50 will experience an osteoporosis-related fracture in their remaining lifetime.2
|
|

|

After an initial fragility fracture, ~1 in 3 female Medicare beneficiaries ≥ 65 years of age experience an additional fracture within 5 years.3,†
|
|
|
|
|
Postmenopausal women face a 5x greater chance of suffering another fracture within the first year of an osteoporosis-related fracture, and the risk remains elevated over time.4,‡
†Data from female Medicare beneficiaries with evidence of fracture: 100% sample for 2010-2012, 5% sample for 2006-2009.
‡Study results from 4140 postmenopausal women, in the Netherlands, with a known fracture history.
|
|
|
|
|
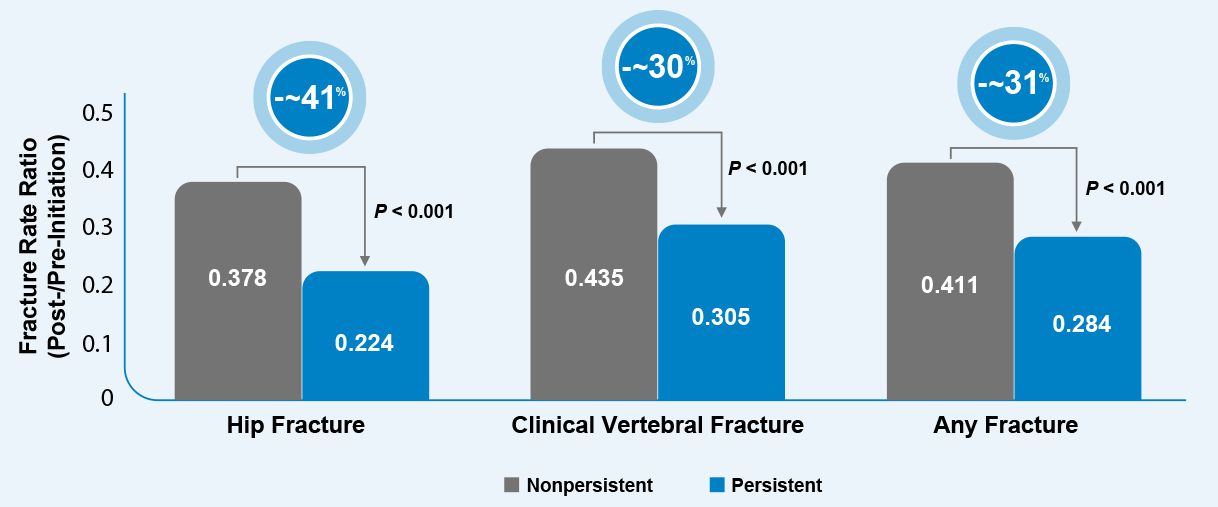
Patients who were persistent on medication had an ~41% greater decline in hip fracture rates compared with patients who were nonpersistent.1,§
|
|
|
|
|
Reduction of Fracture Rate Ratio (FRR) Between Persistent and Nonpersistent Patients1,§
|
|
|
|
|
|
§The study examined the relationship between persistent osteoporosis medication use and fracture risk among elderly female Medicare beneficiaries aged ≥ 66 years (n = 294,369) diagnosed with osteoporosis using Medicare claims January 1, 2009, to June 30, 2011. Persistent medication use was defined as continuous use (no gap ≥ 60 days) for 1 year or longer. (Includes any prescription osteoporosis medication FDA approved through December 2011.)
|
|
|
|
|
|
Persistent use of osteoporosis medication was associated with reduced risk of fracture and significantly lower total healthcare costs.1
|
|
|
|
|
Aim to improve persistence to osteoporosis medications to help your members reduce their risk of fracture and associated healthcare costs.1
|
|
|
|
References: 1. Liu J, Guo H, Rai P, Pinto L, Barron R. Medication persistence and risk of fracture among female Medicare beneficiaries diagnosed with osteoporosis. Osteoporos Int. 2018;29:2409-2417. 2. U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General, 2004. 3. Balasubramanian A, Zhang J, Chen L, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int. 2019;30:79-92. 4. Van Geel TACM, Van Helden S, Geusens PP, Winkens B, Dinant G-J. Clinical subsequent fractures cluster in time after first fracture. Ann Rheum Dis. 2009;68:99-102.
|
|
|
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799
www.amgen.com
© 2020 Amgen Inc. All rights reserved.
USA-785-81449 11/20
|
|
|
|
|
|
|